- Home
- I am a …
- Resources
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- More …
- Literacy in science teaching
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Collections
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
- More from navigation items
- Hot topic:
- RSC members may be unable to sign in to our website. We are working to resolve this as soon as possible.
Close menu
- Home
- I am a …
-
Resources
- Back to parent navigation item
- Resources
- Primary
- Secondary
- Higher education
- Curriculum support
- Practical
- Analysis
- Literacy in science teaching
- Periodic table
- Climate change and sustainability
- Careers
- Resources shop
-
Collections
- Back to parent navigation item
- Collections
- Post-lockdown teaching support
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- More …
- Literacy in science teaching
- Climate change and sustainability
- Alchemy
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
-
Chemistry and art
- Back to parent navigation item
- Chemistry and art
- Techniques
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Cave art
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- Events
- Teacher PD
- Enrichment
- Our work
Resources
Browse our wealth of support for teaching primary, secondary and higher education students
New resources
Fermentation of glucose using yeast | 14–16 years
In association with Nuffield Foundation, By Neil Goalby
The sublimation of air freshener | 11–14 years
In association with Nuffield Foundation, By Dorothy Warren
The ‘breathalyser’ reaction | 16–18 years
In association with Nuffield Foundation, By Tim Jolliff and Sandrine Bouchelkia
Fermentation of glucose using yeast | 14–16 years
The sublimation of air freshener | 11–14 years
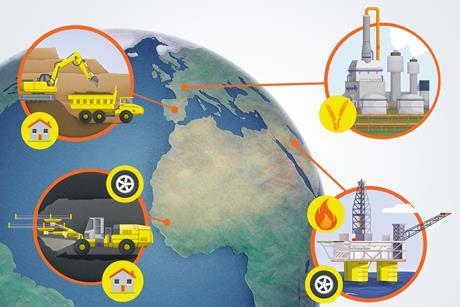
Teaching Earth’s resources
Covalent bonding | Structure strip | 14–16
Ionic bonding | Structure strip | 14–16
Metallic bonding | Structure strip | 14–16
Allotropes of carbon | Structure strip | 14–16
Chromatography challenge | 16–18 years
The ‘breathalyser’ reaction | 16–18 years
- Previous
- Next
New collections
Review my learning worksheets | 14–16 years
Differentiated worksheets to assess learners’ understanding and misconceptions in key topics
Chemistry for All project resources
Practical resources used by some of the activity providers for outreach work as part of the Chemistry for All project
Primary
Free classroom resources, videos and experiments to support your teaching of primary school science
Secondary
Over 1800 resources to save you time, spark excitement and encourage understanding
Higher education
Support your students with the skills they need to succeed at degree level and beyond
Practical
Resources and inspiration for experiments and demonstrations
Analysis
Explore the principles and practice of spectroscopy and other analytical methods
Periodic table
Explore the elements with your students using these innovative and interactive resources
Education Prizes 2024
It’s time to recognise the teams, collaborations and individuals making an impact in science education
More collections
- Previous
- Next