

|
Received (in Cambridge) 18th March 1999 , Accepted 10th June 1999
The first example of an enzyme catalysed modification of the backbone of a synthetic polymer is described. An immobilised lipase from Candida antarctica (Novozym 435) catalyses the selective epoxidation of polybutadiene in organic solvents in the presence of hydrogen peroxide and catalytic quantities of acetic acid. The cis and trans alkene bonds of the backbone are epoxidised in yields of up to 60% whilst the pendent vinyl groups are untouched. The effect of varying a number of reaction parameters suggests that higher yields of epoxide could not be obtained because of the conformational properties of the partially epoxidised polymer. Application of this same enzymatic process to the Baeyer–Villiger reactions of poly(phenyl vinyl ketone) and poly(methyl vinyl ketone) were unsuccessful. The lack of reactivity was found to be a property of the polymers rather than of the enzymatic system.
Enzymes are becoming increasingly important in organic chemistry. They have the advantage of being environmentally benign, highly selective and extremely active catalysts which are able to catalyse a variety of reactions under mild conditions. The ability of enzymes to function in organic solvents has greatly increased their versatility. 1 Biotransformations have been particularly important in the synthesis of chiral compounds as building blocks for organic synthesis. 2 Many of these compounds would be difficult to prepare with conventional chemical techniques. Whilst there is a large body of work relating to biotransformations in organic synthesis, there are far fewer instances of enzymes being used in polymer synthesis. There are three main areas of polymer synthesis in which enzymes have been applied. 3 The first, and by far the largest of these, is the use of hydrolases in organic solvents as catalysts for polyester synthesis. In particular lipases have been shown to catalyse polyesterification, 4 polytransesterification 5 and ring opening polymerisation. 6 These enzymatic processes often require reaction times of several days to achieve high molecular weight polymers. The stereoselectivity of enzyme catalysed reactions has been exploited to prepare optically active polyesters from racemic starting materials, 7 whilst the regioselectivity of enzymes has been utilised in a one step synthesis of a sucrose containing polyester, without the need for any protecting groups. 8 Enzymes have also been used in oxidation polymerisations 9 and the in vitro synthesis of polysaccharides. 10 A second, and far smaller, area in which enzymes have been employed is the synthesis of monomers. The high degree of stereo- and regio-selectivity in enzyme catalysed reactions has been applied to good effect in the concise synthesis of chiral monomers from racemic starting materials, 11 and in the synthesis of radically polymerisable carbohydrate derivatives, without the need for protecting groups. 12 The third, and by far the smallest of the three areas, in which enzymes have been used to advantage in polymer synthesis is the modification of preformed synthetic polymers. Although the ability of enzymes to hydrolyse certain synthetic polymers has been known for many years 13 there are remarkably few examples of enzymes being used to modify synthetic polymers via bond forming reactions. Ritter, has reported the enzyme catalysed esterification of a hydroxy or carboxylic acid group near the end of a long polymer side-chain, 14 however an enzyme catalysed modification of the backbone of a synthetic polymer has not been observed.
The epoxide group is one of the most versatile functional groups in organic synthesis. This is due largely to its reactions with a wide range of nucleophiles, resulting in ring opening almost always with high stereoselectivity and often with high regioselectivity. In addition, these transformations are rarely complicated by competing elimination reactions. There are reports of the enzyme catalysed introduction of the epoxide group into small molecules. 15–17 Given the versatility of the epoxide group in organic synthesis and the commercial importance of unsaturated polymers, in the form of natural and synthetic rubbers, it seemed to us that it would be of interest to examine the enzyme catalysed introduction of the epoxide group into polymer systems.
Alkenes have been epoxidised by mono-oxygenase enzymes. 15 However, there are a number of disadvantages to the use of these systems. These isolated enzymes are relatively unstable and require cofactors and several proteins to act in a concerted fashion. 15 The use of whole cells is an alternative, however this approach also has disadvantages. These organisms might have difficulty assimilating the large molecules of synthetic polymers and expensive cofactors, such as NADPH (nicotinamide adenine dinucleotide phosphate, reduced form), would need to be recycled. Also, whole cells could not be used in organic solvents and only a limited range of polymers could be studied. The isolation of the modified polymer could be difficult as it would need to be separated from the cell debris, much of which is also polymeric. The only isolated enzyme available for the epoxidation of alkenes is chloroperoxidase from Caldariomyces fumago. 16 Although this enzyme does not require cofactors, it operates in aqueous buffer, limiting the number of suitable polymeric substrates which could be investigated. A potentially more useful approach takes advantage of work by Björkling and co-workers in which an immobilised lipase isolated from Candida antarctica was used to catalyse the epoxidation of alkenes in organic solvents, using hydrogen peroxide as an oxidant and catalytic quantities of a medium chain alkanoic acid. 17 The enzyme was found to catalyse the in situ synthesis of the corresponding peracid which then epoxidised the alkene. The enzyme played no part in the actual epoxidation step. Roberts and co-workers later reported that this same system could be used for Baeyer–Villiger reactions. 18 The simplicity of this system and the fact that the enzyme did not have to interact directly with the polymer substrate suggested that it might be possible to modify polymers by this process. The epoxidation of polybutadiene and the Baeyer–Villiger reactions of poly(phenyl vinyl ketone) and poly(methyl vinyl ketone) were chosen for our initial investigations. We now wish to report the first successful enzyme catalysed modification of the backbone of a synthetic polymer. 19
Initial experiments were carried out to investigate the possibility of epoxidising monophenyl terminated polybutadiene (Mn 1300) (35% trans, 20% cis, 45% vinyl) using the method of Björkling. 17 The reaction was carried out for 96 hours in dichloromethane at 25 °C with 10 mol% of acetic acid, 10 wt% of Candida antarctica lipase (Novozym 435) and a 27.5 wt% aqueous solution of hydrogen peroxide, see Scheme 1.

Scheme 1 Reagents and conditions: (i) CH3CO2H, H2O2(aq), Novozym 435, CH2Cl2.
The 1H NMR spectra of the polybutadiene and the epoxidised product are shown in Fig. 1, whilst the 13C NMR spectra are shown in Fig. 2. The new signals in the spectra of the product are consistent with formation of partially epoxidised polybutadiene. 20 The phenyl terminating group can be used to determine the extent of epoxidation and the selectivity as it is unchanged by the reaction. Each polymer chain is terminated with one phenyl group containing five protons, therefore, the area corresponding to one proton can be calculated from the 1H NMR spectra. This allows the number of vinyl [z.dbd6 ]CH2 protons to be calculated and therefore the number of vinyl [z.dbd6 ]CH– protons. The cis and trans–CH[z.dbd6 ]CH– proton signals overlap with those of the vinyl [z.dbd6 ]CH– protons but their number can be calculated by simple subtraction. Elemental analysis of the epoxidised polymer suggested that 30% of the alkene bonds had been epoxidised. This value is in close agreement with the 32% calculated from the 1H NMR spectrum. Obviously, the elemental analysis gives only a total value for the yield of epoxide and no indication of the nature of the alkene bonds which had been epoxidised. Although elemental analysis was used to confirm the NMR results, and in many cases the results were in good agreement, there were also cases where the agreement was poor due, it is thought, to the retention of solvent in the viscous products. As the NMR analysis gave more information about the selectivity of the reaction and was reproducible, this technique was routinely used to analyse the products. The epoxidation of polybutadiene under these mild conditions was found to be highly selective. It is apparent from the 1H NMR that only the cis and trans alkene bonds had been epoxidised leaving the vinyl alkene bonds completely untouched. As the composition of the polymer was known, it was established that 59% of the alkene bonds in the polymer backbone had been epoxidised. Some selective preference for epoxidation of the alkene bonds of the backbone (cis > trans > vinyl) has been observed in previous attempts to epoxidise polybutadiene chemically. 20,21 In these systems the vinyl groups began to react before all of the backbone alkene bonds had reacted and some of the backbone alkene bonds remained unreacted. Small amounts of ring opened products were also observed in the epoxidation of polybutadiene with a 1∶2 mixture of acetic acid and 60 wt% hydrogen peroxide. 21b Only one approach, using a molybdenum catalyst, has shown both high selectivity and complete conversion. 22 With this catalyst the backbone alkene bonds were completely epoxidised within three hours at room temperature and there was no further change in the next 70 hours. The mild conditions of the enzymatic epoxidation procedure allows the selective epoxidation of the backbone alkene bonds without opening the epoxide rings. No epoxidation of vinyl groups was observed. Gel permeation chromatography (GPC) revealed that the molecular weight of the polymer was not significantly altered in our enzyme catalysed reaction suggesting that no chain scission or crosslinking had taken place.

Fig. 1 1H NMR spectra of a) polybutadiene b) epoxidised polybutadiene.

Fig. 2 13C NMR spectra of a) polybutadiene b) epoxidised polybutadiene.
Once a basic reaction procedure had been established, the effect of varying a number of reaction conditions was investigated. The results shown in Table 1 are the results of individual experiments since it is difficult to sample multiphase systems accurately. Control experiments showed that no reaction took place in the absence of enzyme catalyst.
|
Entry |
Time/h |
Acid (mol%) |
Enzyme (wt%) |
Solvent |
Temperature°C |
Concentration H2O2 (wt%) |
Yield of epoxide (elemental analysis) (%) |
Yield of epoxide (1H NMR) (%) |
Yield of epoxide cis–trans (1H NMR) (%) |
|
a
Added in one portion at the start of the reaction. bAdded dropwise over the course of the reaction. c10 wt% added and the reaction stirred for 24 hours. A second 10 wt% was then added and the reaction allowed to proceed for a further 24 hours. dThe enzyme used was recovered from a previous epoxidation reaction. |
|||||||||
| 1 | 2 | Acetic (10) | 10 | CH2Cl2 | 25 | 27.5 | 9 | 0 | 0 |
| 2 | 6 | Acetic (10) | 10 | CH2Cl2 | 25 | 27.5 a | 14 | 13 | 24 |
| 3 | 24 | Acetic (10) | 10 | CH2Cl2 | 25 | 27.5 a | 37 | 30 | 54 |
| 4 | 96 | Acetic (10) | 10 | CH2Cl2 | 25 | 27.5 a | 30 | 32 | 59 |
| 5 | 24 | Acetic (5) | 10 | CH2Cl2 | 25 | 27.5 a | — | 21 | 38 |
| 6 | 24 | Acetic (20) | 10 | CH2Cl2 | 25 | 27.5 a | — | 14 | 25 |
| 7 | 24 | Palmitic (10) | 10 | CH2Cl2 | 25 | 27.5 a | — | 14 | 26 |
| 8 | 24 | Octanoic (10) | 10 | CH2Cl2 | 25 | 27.5 a | — | 33 | 60 |
| 9 | 24 | Trifluoroacetic (10) | 10 | CH2Cl2 | 25 | 27.5 a | — | 31 | 57 |
| 10 | 24 | Acetic (10) | 10 | CH2Cl2 | 25 | 27.5 b | 14 | 12 | 22 |
| 11 | 24 | Acetic (10) | 10 | CH2Cl2 | 25 | 13.75 a | 39 | 30 | 55 |
| 12 | 24 | Acetic (10) | 10 | CH2Cl2 | 25 | 13.75 b | 13 | 12 | 22 |
| 13 | 24 | Acetic (10) | 10 | Toluene | 25 | 27.5 a | — | 24 | 44 |
| 14 | 24 | Acetic (10) | 10 | Toluene | 25 | 27.5 b | 26 | 32 | 58 |
| 15 | 24 | Acetic (10) | 10 | Hexane | 25 | 27.5 a | — | 0 | 0 |
| 16 | 24 | Acetic (10) | 10 | Hexane | 25 | 27.5 b | 7 | 3 | 5 |
| 17 | 24 | Acetic (10) | 10 | CH2Cl2 | 37 | 27.5 a | 12 | 10 | 19 |
| 18 | 24 | Acetic (10) | 10 | CH2Cl2 | 37 | 27.5 b | 9 | 8 | 14 |
| 19 | 24 | Acetic (10) | 10 | Toluene | 37 | 27.5 b | 19 | 18 | 33 |
| 20 | 24 | Acetic (10) | 10 | Hexane | 37 | 27.5 b | 7 | 5 | 9 |
| 21 | 24 | Acetic (10) | 5 | CH2Cl2 | 25 | 27.5 a | 0 | 21 | 38 |
| 22 | 24 | Acetic (10) | 20 | CH2Cl2 | 25 | 27.5 a | 37 | 30 | 54 |
| 23 | 24 | Acetic (10) | 5 | CH2Cl2 | 25 | 27.5 b | 13 | 11 | 20 |
| 24 | 24 | Acetic (10) | 20 | CH2Cl2 | 25 | 27.5 b | — | 14 | 26 |
| 25 | 24 | Acetic (10) | 20 c | CH2Cl2 | 25 | 27.5 b | 29 | 28 | 51 |
| 26 | 24 | Acetic (10) | 10 d | CH2Cl2 | 25 | 27.5 b | 2 | 0 | 0 |
It is apparent from Table 1 (entries 1–4) that the reaction is essentially complete after 24 hours. This is almost certainly because the enzyme becomes oxidised and inactive during the course of the reaction. Entry 26, which used enzyme which had been recovered from a previous epoxidation reaction, shows that the enzyme was completely inactive and no epoxidation occurred.
In the initial enzyme catalysed epoxidation experiments of polybutadiene 10 mol% of acetic acid was used for each mole of alkene bonds present in polybutadiene. It was thought that increasing the quantity of acetic acid would increase the rate of formation of peroxyacetic acid and therefore increase the rate of epoxidation, allowing a higher degree of epoxidation to occur before the enzyme became inactive. Surprisingly, it was found by 1H NMR analysis that a lower yield of epoxide was obtained both with a higher concentration of acetic acid (20 mol%; entries 3 and 6) and with a lower concentration (5 mol%; entries 3 and 5), see Table 1. One possible explanation for this observation is that higher concentrations of acetic acid may increase the rate of enzyme deactivation, whilst at the lower concentration the rate of epoxidation is reduced and the enzyme becomes deactivated at a lower epoxide conversion.
Björkling used long-chain carboxylic acids in the development of this enzyme catalysed epoxidation procedure. 17 We found that when palmitic acid was used in place of acetic acid lower yields of epoxide were obtained, see Table 1 entries 3 and 7. Palmitic acid produced emulsions during the work up procedure and consequently lower yields were obtained. Octanoic acid also produced emulsions, but these were easier to break and yields comparable to those with acetic acid were obtained, see Table 1 entries 3 and 8. The easier work up procedure for acetic acid was therefore advantageous. Trifluoroperoxyacetic acid is often the acid of choice in epoxidation reactions due to its high reactivity. Reactions using trifluoroacetic acid gave similar results to those using acetic acid with no increase in epoxide formation, see Table 1 entries 3 and 9.
Björkling found that addition of hydrogen peroxide over the course of the reaction increased product yield by allowing the enzyme to remain active longer. 17 High hydrogen peroxide concentrations rapidly damage the enzyme by oxidation denaturing processes. Low hydrogen peroxide concentrations are achievable by either using a more dilute solution of hydrogen peroxide, or by the Björkling method of addition over the duration of the reaction. Slow addition of hydrogen peroxide enables the enzyme to consume the oxidising agent at a rate which minimises its exposure to unreacted hydrogen peroxide. It can be seen from Table 1 that alterations in the concentration of the hydrogen peroxide had little effect on the yield of epoxide (entries 3, 10–12). In contrast, lower yields of epoxide were obtained when the oxidant was added slowly during the course of the reaction rather than in one portion at the start. The lower yields obtained with the dropwise addition of hydrogen peroxide may be attributed to the low rates of epoxidation caused by the very low concentration of peroxyacid in the system.
Comparison of entries 3, 10, 13 and 14 in Table 1 show that in toluene a higher yield of epoxide was obtained when the hydrogen peroxide was added dropwise over the course of the reaction rather than in one portion at the beginning, whereas with dichloromethane the opposite effect was observed. Presumably, the relative rates of denaturation and epoxidation are altered in toluene. The maximum yield of epoxide was almost identical with both toluene and dichloromethane although the method of hydrogen peroxide addition differed. Hexane had been reported to be an excellent solvent for this enzymatic system 17 but surprisingly no appreciable reaction in hexane was observed (entries 15 and 16). One difference between our system and that of Björkling was that we used acetic acid whereas he used long chain acids. 17 It is possible that the lower solubility of peroxyacetic acid in hexane compared with peroxypalmitic acid could be an important factor.
It was found that increasing the temperature from 25 °C to 37 °C reduced the yield of epoxide in both dichloromethane and toluene whilst again little reaction was observed in hexane, see Table 1 entries 3, 10, 14, 16–20. It is also apparent that the yield of epoxide is decreased both when the oxidant is added in one portion at the beginning of the reaction and when it is added dropwise throughout the course of the reaction. There could be two possible explanations for the decrease in the yield of epoxide as the temperature is raised. The higher temperature could be either increasing the rate at which the enzyme is being denatured, or alternatively it could be accelerating the decomposition of the hydrogen peroxide. Both of these eventualities would decrease the levels of the epoxide formed. However, oxidant was still detectable (sodium metabisulfate and starch and potassium iodide test strips) in the reaction mixtures after each reaction. Since the actual concentration of the hydrogen peroxide was found to have little effect on the yield of epoxide it is likely that an increase in the rate of denaturation is responsible for the lower yield of epoxide.
Given that the enzyme is crucial to the in situ synthesis of peroxyacetic acid, it was logical to investigate the optimal quantities of enzyme catalyst required in the system whilst keeping other reaction variables constant. Doubling the quantity of enzyme would be expected to increase the rate of reaction. It seemed likely that this higher reaction rate would lead to a higher yield of epoxide before the enzyme became inactive. The yields of epoxide were virtually unchanged if the quantity of enzyme was altered and the hydrogen peroxide added over the course of the reaction, see Table 1 entries 10, 23 and 24. However, it was found that when the hydrogen peroxide was added at the beginning of the reaction and the quantity of Novozym 435 reduced from 10 wt% to 5 wt% the percentage of epoxidation was reduced from 30% to 21% (entries 3, and 21). This lower yield of epoxide can be explained by a lower reaction rate since the amount of epoxide formed before the smaller quantity of enzyme became inactive would be expected to be lower. In contrast, the yield of epoxide was unchanged on increasing the quantity of enzyme from 10 wt% to 20 wt% (entries 3 and 22). It is unclear why the yield of epoxide is essentially unchanged when the quantity of enzyme is doubled. A possible explanation is that there is no oxidant remaining after 24 hours. This explanation can be discounted as oxidant could be detected (sodium metabisulfate and starch and potassium iodide test strips) after each reaction. Also, addition of a second 10 wt% of enzyme after 24 hours and allowing the reaction to proceed for a further 24 hours resulted in a substantial increase in the yield of epoxide (entries 10 and 25). For the first portion of the enzyme the hydrogen peroxide was added dropwise over the course of the reaction and it is apparent from Table 1 entry 10 that this reaction gives a polymer with 12% of its alkene bonds epoxidised. Given that the enzyme becomes completely deactivated within 24 hours the second portion of enzyme would be in a similar system to that where the oxidant is added in one portion at the beginning of the reaction. Entry 3 shows that under these conditions 30% of the alkene bonds should be epoxidised. It would therefore be expected that the total yield of epoxide would be approximately 42%. Our results showed that only 28% of the alkene bonds were in fact epoxidised. This value is very similar to the maximum yield of 32% that has been obtained with this enzyme catalysed process (entries 3, 4, 8, 11, 14, and 22). These results suggest that the alkene bonds of the polymer are no longer available for epoxidation due to conformational changes of the polymer during the epoxidation reaction. Given that the system is a well mixed combination of an aqueous phase and an organic phase (in which the polymer is dissolved), it seems probable that the epoxidised polymer would adopt conformations in which the more hydrophilic epoxide groups are able to interact with the aqueous phase whilst the hydrophobic alkenes are buried within the centre of the polymer molecule. This would have the effect of rendering unepoxidised alkene groups inaccessible for further reactions. In support of this, Zuchowska reported that the epoxidation of polybutadiene with concentrated acetic acid and 60 wt% aqueous hydrogen peroxide resulted in a decrease in rate of epoxidation as the reaction proceeded. 21b It was suggested that side reactions involving ring opening were responsible for the rate decrease with time. It is unlikely that this explanation is applicable to our system as very mild reaction conditions were used and FT-IR, 1H NMR and 13C NMR analysis showed no evidence of ring opening. Conformational changes appear to be a more likely explanation for the cessation of the reaction in our case.
To demonstrate the importance and benefits of the mild enzyme catalysed system, polybutadiene was epoxidised with a 32 wt% solution of peroxyacetic acid in acetic acid in dichloromethane at 25 °C. It was found that the cis, trans, and vinyl alkene bonds were completely epoxidised. FT-IR spectra of the products showed evidence of ring opened products [ max(thin film)/cm−1: 3445 (OH) and 1736 (ester)]. There was no evidence of residual acid being retained by the polymer. Interestingly, the epoxide groups of polyepoxide polymers derived from polybutadiene have been reported to be relatively inert to ring opening reactions with carboxylic acids, requiring forcing conditions.
23
The mild reaction conditions, the selectivity of the epoxidation reaction and the ability to generate the potentially hazardous peracetic acid in situ are obvious advantages of the enzyme catalysed process.
max(thin film)/cm−1: 3445 (OH) and 1736 (ester)]. There was no evidence of residual acid being retained by the polymer. Interestingly, the epoxide groups of polyepoxide polymers derived from polybutadiene have been reported to be relatively inert to ring opening reactions with carboxylic acids, requiring forcing conditions.
23
The mild reaction conditions, the selectivity of the epoxidation reaction and the ability to generate the potentially hazardous peracetic acid in situ are obvious advantages of the enzyme catalysed process.
Roberts and co-workers exploited the lipase-catalysed peroxyacid formation, developed by Björkling, in Baeyer–Villiger reactions. 18 Roberts was able to show that Baeyer–Villiger oxidation of cyclic ketones using this approach took place with only slightly reduced yields (typically only 5–10% smaller) compared with those obtained with m-chloroperoxybenzoic acid. Since we had used this system successfully for the epoxidation of polybutadiene, it was thought that it might also be applicable to the Baeyer–Villiger oxidation of poly(phenyl vinyl ketone) and poly(methyl vinyl ketone).
The migratory aptitude of the phenyl group and the secondary alkyl group in poly(phenyl vinyl ketone) are similar. A good model system for this reaction is the Baeyer–Villiger oxidation of phenyl isopropyl ketone which gives a mixture of the two esters. 24 The ester in which the isopropyl migrates predominates, this ester being formed in twice the yield of the other ester. Scheme 2 shows the two possible products from the Baeyer–Villiger oxidation of poly(phenyl vinyl ketone).

Scheme 2 Reagents and conditions: (i) CH3CO2H, H2O2(aq), Novozym 435, CH2Cl2.
The Baeyer–Villiger reaction of poly(phenyl vinyl ketone) was carried out for 24 hours in dichloromethane at 25 °C with 10 mol% acetic acid, 10 wt% Candida antarctica lipase (Novozym 435) and a 27.5 wt% aqueous solution of hydrogen peroxide added in one portion at the start of the reaction. The 1H NMR spectra of poly(phenyl vinyl ketone) and its oxidation products were extremely broad and no useful information about the outcome of the reaction could be obtained. However, examination of the 13C NMR spectra showed the appearance of a second signal at 133.0 ppm, see Fig. 3. Several of the reaction parameters were varied to try to increase the size of this signal. The effect of time was investigated by carrying out reactions for 2, 6, 24 and 96 hours. The effect of hydrogen peroxide concentration was studied by decreasing the concentration of the hydrogen peroxide from 27.5 to 13.75 wt%. A reaction using trifluoroacetic acid in place of acetic acid was also carried out. Finally a reaction in which the amount of Novozym 435 was increased from 10 to 20 wt% was also studied. All of these reactions gave products which had almost identical 13C NMR spectra. In addition, control reactions carried out without the enzyme also gave identical results. Surprisingly, the product obtained from a chemical Baeyer–Villiger reaction, using a 32 wt% solution of peracetic acid in acetic acid as oxidant, also gave a product with a similar 13C NMR spectrum. Whilst the new signal in the 13C NMR spectra of the products might be attributed to the formation of ester from a Baeyer–Villiger reaction, infrared spectra of the products showed no evidence of ester carbonyl groups.

Fig. 3 13C NMR spectra of a) poly(phenyl vinyl ketone) b) modified poly(phenyl vinyl ketone).
The common feature of all of these reactions is the presence of acid and water. It seems likely that the reaction which occurs is catalysed by acid. Surprisingly, attempts to carry out Baeyer–Villiger reactions with poly(methyl vinyl ketone) using the same reaction conditions resulted in isolation of the unchanged polymer. It is apparent from these studies that the lack of reactivity is due to the nature of the polymers rather than the enzyme catalysed system.
It was found that an immobilised enzyme from Candida antarctica can be used to selectively epoxidise polybutadiene in organic solvents under very mild conditions. This is the first example of an enzyme catalysed modification of the backbone of a synthetic polymer. The reaction shows a high selectivity for the cis and trans alkene bonds of the polymer backbone leaving the pendent vinyl groups untouched. It was found that up to 60% of the cis and trans alkene bonds could be epoxidised. The effect of varying a number of parameters was investigated but higher yields of epoxide could not be obtained. These results showed that the effect of varying even a single parameter did not lead to a readily predictable outcome. This is probably due to the complex nature of this three phase system in which several interdependent processes are occurring simultaneously. However, these results do suggest that the inability to obtain higher yields of epoxide is probably due to changes in the conformational properties of the partially epoxidised polymer.
Attempts to use the same enzyme catalysed system to carry out Baeyer–Villiger oxidations of poly(phenyl vinyl ketone) and poly(methyl vinyl ketone) were unsuccessful. Poly(phenyl vinyl ketone) was modified but in a non-enzymatic process. Given that similar results were obtained in the absence of enzyme and in a conventional chemical reaction with peracetic acid it can be concluded that the lack of reactivity is a property of the polymers and not of the enzymatic reaction.
All materials were purchased from the Aldrich Chemical Co. and were used as received. Novozym 435 was a gift from Novo Nordisk. IR spectra were recorded on a Perkin-Elmer 1710 Fourier Transform infrared spectrometer. Liquids were prepared as thin films between sodium chloride plates and solids cast as films from solvent onto sodium chloride plates. NMR spectra were recorded on a Bruker AC 300 spectrometer. 13C NMR spectra were recorded as PENDANT spectra. Elemental microanalyses were performed by Medac Ltd, Department of Chemistry, Brunel University, Uxbridge, Middlesex. Whilst the percentage of oxygen in these samples could not be obtained directly from the C, H, N analysis, comparison with the data obtained for the unreacted polybutadiene allowed the percentage of oxygen to be estimated by difference. Gel permeation chromatography was performed by RAPRA Technology Ltd., Shawbury, Shrewsbury, Shropshire, UK SY4 4NR. Analysis was carried out at 30 °C with a PL Gel 10μ 2 × mixed bed-B column calibrated with polystyrene standards, tetrahydrofuran as solvent, a flow rate of 1.0 cm3 min−1 and a refractive index detector.
A typical procedure for both epoxidation and Baeyer–Villiger reactions is as follows: polybutadiene (5.0 g, 92 mmol of alkene bonds) was dissolved in dichloromethane (100 cm3). Acetic acid (0.23 cm3, 9 mmol), a 27.5 wt% aqueous solution of hydrogen peroxide (17.0 cm3, 0.14 mol), and Novozym 435 (0.5 g) were added and the mixture stirred for 24 hours in the dark. The mixture was then filtered and extracted with a saturated aqueous solution of sodium hydrogen carbonate and dried over magnesium sulfate. The solvent was removed and the polymer dried in a vacuum oven at 55 °C at 1 mmHg.
The general procedure was followed but Novozym 435, acetic acid and hydrogen peroxide were omitted. A 32 wt% solution of peroxyacetic acid in acetic acid (16.4 cm3, 70 mmol) was added and the mixture stirred for 96 hours, yielding the product as an opaque viscous liquid (5.3 g);  max/cm−1 3445s (OH), 1736s (ester C[z.dbd6 ]O);
max/cm−1 3445s (OH), 1736s (ester C[z.dbd6 ]O);  H (300 MHz, CDCl3) 1.0–2.7 (br m, -CH2- & -CH3), 3.0–4.6 (br m, -CH-), 7.0–7.2 (aromatic CH);
H (300 MHz, CDCl3) 1.0–2.7 (br m, -CH2- & -CH3), 3.0–4.6 (br m, -CH-), 7.0–7.2 (aromatic CH);  C (75 MHz, CDCl3) 20.9, 29.6, 81.6, 111.0, 125.7, 128.3.
C (75 MHz, CDCl3) 20.9, 29.6, 81.6, 111.0, 125.7, 128.3.
Methyl vinyl ketone (41.6 cm3, 0.50 mol) was dissolved in methanol (100 cm3). The solution was degassed for 30 minutes by bubbling argon though the mixture. 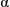 ,
,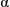 ′-Azobis(isobutyronitrile) (0.82 g, 5.0 mmol) was added and the reaction mixture refluxed for 24 hours under an argon blanket. The reaction mixture was allowed to cool and poured into water (500 cm3) giving a white solid. The majority of the solvent was decanted off and the solid filtered off and washed with methanol. The resultant poly(methyl vinyl ketone) was dried over anhydrous silica gel in a vacuum oven at 40 °C/1 mmHg for 24 hours giving the polymer as a white solid (31.2 g, 89%); Mn 6270, Mw 13700;
′-Azobis(isobutyronitrile) (0.82 g, 5.0 mmol) was added and the reaction mixture refluxed for 24 hours under an argon blanket. The reaction mixture was allowed to cool and poured into water (500 cm3) giving a white solid. The majority of the solvent was decanted off and the solid filtered off and washed with methanol. The resultant poly(methyl vinyl ketone) was dried over anhydrous silica gel in a vacuum oven at 40 °C/1 mmHg for 24 hours giving the polymer as a white solid (31.2 g, 89%); Mn 6270, Mw 13700;  H (300 MHz, CDCl3) 0.91–2.50 (br m);
H (300 MHz, CDCl3) 0.91–2.50 (br m);  C (75 MHz, CDCl3) 29.2, 32.6, 47.8, 210.5.
C (75 MHz, CDCl3) 29.2, 32.6, 47.8, 210.5.
We thank the EPSRC for a studentship (to N.O.) and Novo Nordisk for the gift of Novozym 435.
Paper 9/02165E
P1RUnknown Journal Code 1999, 15, 2171- 2176