Brush up on Avogadro’s number and be prepared to explore some questions that put learners problem-solving to the test
Build upon learner’s understanding of catalysts and enzymes using several open-ended questions.
Introduction
Teachers who have not used the problems before should read the section Using the problems before starting.
Prior knowledge
General knowledge about catalysts and enzymes, and Avogadro’s number. A detailed knowledge is unnecessary as students are encouraged to consult textbooks and data books during the exercise.
Resources
Scientific calculators, data books and textbooks for reference.
Possible solutions
Question (i) requires the exercise of judgement and the ability to make sensible ‘guesstimates’. Students are generally not accustomed to this in a scientific context and may feel uncomfortable, but push them into guessing suitable figures. Get them to think; give them help when required, but only as much as is necessary.
Questions (ii) and (iii) involve straightforward arithmetic while question (iv) does not involve any calculation.
(i) Students are forced into making an assumption (guess) based upon inadequate information – 1 g of blood on the handkerchief is assumed in the following calculation.
If 1 g of blood contains 0.0001 g catalase then there is (0.0001/33000) mole
catalase or (0.0001/33000) x 6.0 x 1023 molecules of catalase
= 1.8 x 1015 molecules.
(ii) Number of peroxide molecules decomposed per minute
= 1.8 x 1015 x 5 x 106 = 1022.
Compare these figures with:
- the UN estimate for the 1990 world population = 5.25 x 109;
- the number of cells in a 12-stone man = 1014; and
- the number of cells in the largest living creature, the blue whale = 1017.
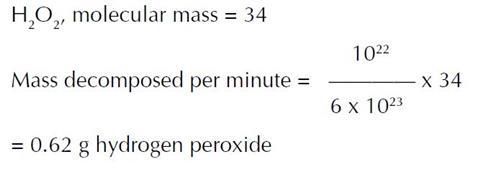
(i) 50 g detergent, 2 % hydrogen peroxide, ie 1 g of hydrogen peroxide. This would last between 1–2 minutes.
(ii) The problem of enzyme deactivation would be greatest in Japan where all major brands of detergent contain enzymes. Washing at high temperatures would deactivate both the enzyme in the blood and the enzyme in the detergent although the bleach would still work. Bleach, however, is not as effective as enzymes for biological stains.
A low-temperature prewash would allow the enzymes in the detergent to digest the blood stain, and an ordinary main wash thereafter would allow both the enzymes and the bleach to work.
Other solutions include
- multicomponent detergents. These consist of non-woven rayon sheets on which the different components are deposited in small piles. A second sheet is then laminated on top of the first, keeping the different components isolated. The consumer tosses one or more of these sheets into the washing machine. Until now these have been used to separate fabric conditioner from the other components. The conditioner is encapsulated in wax, and it is the heat of tumble drying that melts the wax and releases the conditioner at the right time; and
- applied biology. Enzymes are inhibited by low molecular weight molecules or by other proteins. It has been observed that tumour tissue from liver does not catalyse the decomposition of hydrogen peroxide as well as normal tissue because the tumour tissue makes a protein that inhibits catalase. The molecular biologist would purify this protein, determine part of its amino acid sequence and use this to clone the gene that encodes the protein responsible for the inhibition. The protein would be over expressed in harmless bacteria such as Escherichia coli. If the protein could be made cheaply enough it might be added to the washing powder to inhibit the catalase present in blood. At present this technology is quite expensive and is used to provide pharmaceutical proteins such as human growth hormone and blood clotting factors that are in short supply. However, the costs of this type of work inevitably reduce with time so that producing a ‘catalase inhibitor’ by this route will eventually become economically viable.
Downloads
Catalase - creative problem-solving
PDF, Size 0.46 mb
Additional information
This resource is part of our Creative problem-solving in chemistry collection.


















No comments yet