This chapter reviews some of the research findings about learners’ ideas about atomic structure and other chemical structures
Introducing some related classroom instruments included in the companion volume.
The structure of the atom
During their secondary education, students are expected to learn about the structure of the atom, or - more correctly - to learn about a particular model of the structure of the atom.
The usual model of the structure of the atom met at this level consists of the nucleus at the centre of one or more shells of electrons. The electrons are usually shown as being placed on these circular shells.
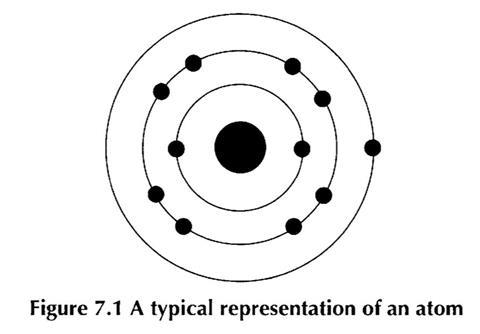
Although this model is perfectly appropriate at this level, those students who take their study of chemistry further will need to accept more detailed models. It is useful, therefore, for secondary teachers to emphasise that such a diagram only represents a model, and is one of several models that together help us understand matter at the atomic scale.
Learners’ ideas about the atomic nucleus
The term ’nucleus’ itself may sound quite similar to ’neutron’ and this may be a source of confusion. More significantly, students will be familiar with the use of ’nucleus’ in biology and may sometimes - hard as it may seem to appreciate - confuse atoms and cells.
In one sense such a comparison is impressive: cells are sometimes considered to be the ’building blocks’ of organisms, and atoms are often said to be the ’building blocks’ of matter. The cell-nucleus-atomic-nucleus analogy can be significant. The cell nucleus is often described as a type of ‘control centre’ for the cell, and the atomic nucleus may be understood to be a control centre for the atom.
Making comparisons between different ideas is an important part of developing new concepts, but learners need to be taught to look for the negative as well as the positive aspects of an analogy. An example of this - seeing the atom as like a tiny solar system.
Learners’ ideas about atomic structure
This lack of application of basic electrical ideas to the atom is reflected in the way students often conceptualise the way the electrons are held in position around the nucleus.
According to accepted scientific principles:
- All electrons in an atom are attracted to the nucleus.
- The force acting on an electron depends upon the magnitude of the nuclear charge and the separation.
- The attractive force between an electron and the nucleus acts in both directions: both experience the same magnitude force.
- Each electron repels the others with a force which depends upon their separation.
To help simplify more complex atoms, we often introduce the idea of ‘shielding’ where inner shell electrons are considered to cancel the effect of an equivalent number of nuclear protons, so we can model the atom as a positive core charge and one shell of outer or valence electrons. This is only partially valid, as the ’electron shells’ are not actually shells and interpenetrate - but it remains a useful approach. However, it is difficult for students to appreciate how the concept of shielding is supposed to work unless they accept the principles above.
Interviews with post-16 students studying chemistry revealed the following alternative conceptions:
- The nucleus is not attracted by the electrons.
- The nucleus attracts an electron more than the electron attracts the nucleus.
- The protons in the nucleus attract one electron each.
- The electrons repel the nucleus.
Learning by analogy - the example of the atom
Meaningful learning relies on the learner interpreting new information in the context of what they already know - so that it ’makes sense’ to them. It was suggested that sometimes when students fail to learn the science that is presented to them, this may be due to understanding differently, when they relate the new material to alternative conceptions they already have.
When the new ideas are too abstract to be directly related to existing ideas, teachers often call upon comparisons with other more familiar contexts. Atomic structure is clearly a topic which is highly abstract, as students are expected to learn about the internal structure of a conjectured entity which is much too small to be directly experienced.
A common comparison that is made is that ’an atom is like a tiny solar system’. The relationship between the nucleus and electrons is here modelled on the sun and planets.
The use of this teaching analogy relies upon a number of assumptions;
- That an atom is in some ways like a solar system
- That the students are familiar enough with the solar system to make use of the comparison
- That the students will recognise in which ways the atom is like a solar system, and in which ways it is not.
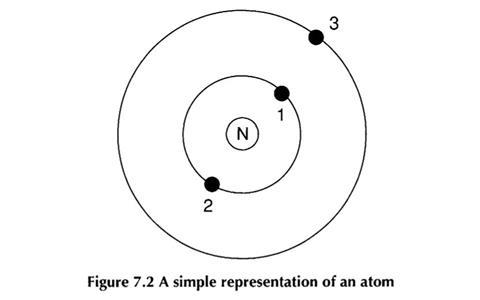
When this was piloted for the project, it was found that students often held alternative ideas about both the atom and the solar system.
Some students thought the electrons could not be interacting with each other, as they were interacting with the nucleus, or there was ’no relationship between them’, whilst others acknowledged a gravitational ’reaction’. Some students did know electrons would repel each other, and one suggested ’they repel each other around the nucleus’.
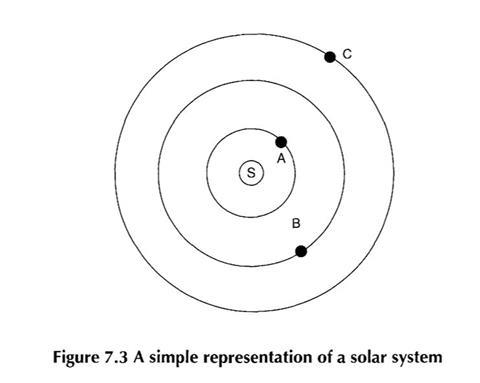
Students were asked to list the similarities and differences between the atomic system and the solar system. Some students found it very difficult to suggest more than a couple of similarities or differences (and many did not make the ’obvious’ point about the atom being a good deal smaller than a solar system).
The class of 14-1 5 year olds which produced the responses discussed above did have a fair attempt at spotting similarities and differences. Some good suggestions were made for both the similarities, and the differences:
Similarities
- Both the atom and the solar system have centres that attract the surrounding planets or electrons.
- Both have forces involved.
- They both rotate around a centre point.
Differences
- More than one thing on the ring in atoms.
- Planets have no charge but electrons are negatively charged.
- Electrons have virtually no mass and planets have a large mass.
- The solar system is a lot bigger.
- Different forces.
- Planets can be seen with the naked eye, electrons can’t.
- The planets rotate around.
Notes
For the full version of this chapter, see downloads below.
Downloads
Chemical structure
PDF, Size 0.68 mb
Websites
Additional information
These PDFs have been taken from the popular book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 1, by Keith Taber

Chemical misconceptions
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
 Currently reading
Currently readingChemical structure
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35





















































No comments yet