Explore what happens when a liquid is supercooled using sodium thiosulfate in this class practical
In this experiment, students melt sodium thiosulfate crystals, before cooling them to a state well below the melting point. The melt now exists in a metastable supercooled state. The supercooled liquid will freeze rapidly on the addition of a crystal of sodium thiosulfate or dust particles, or on stirring. Students add a crystal of the solid to seed the crystallisation process, and observe temperature changes throughout.
This is best carried out individually or in pairs. The experiment takes 20–30 minutes.
Equipment
Apparatus
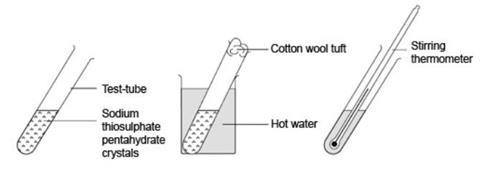
- Test tube (see note 4 below)
- Test tube holder
- Stirring thermometer, –10 °C to 110 °C (see note 5)
- Beaker, 100 cm3
- Bunsen burner
- Tripod and gauze
- Cotton wool, small tuft to fit test tube
Chemicals
- Sodium thiosulfate-5-water, about 20 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Sodium thiosulfate-5-water, Na2S2O3.5H2O(s) – see CLEAPSS Hazcard HC095A.
- The test tube must be very clean.
- A temperature sensor attached to a computer can be used in place of a thermometer. Thus a continuous plot can be drawn as the temperature changes occur.
Procedure
- Half-fill a very clean test tube with crystals of sodium thiosulfate-5-water.
- Put a tuft of cotton wool in the top of the test tube to exclude dust.
- Warm the test tube gently in a beaker of hot water (about 50 °C) to melt the crystals.
- When all the crystals have melted, remove the cotton wool, put a thermometer in the melt and record the temperature. If the liquid starts to crystallise on inserting the thermometer, reheat in water to melt all the solid.
- Stand the test tube in an empty beaker and leave to cool where it won’t be disturbed.
- Observe the temperature at various intervals until the value is in the region of 30–40 °C. No crystallisation should have occurred.
- Now add a fresh crystal of sodium thiosulfate, observe the rapid crystallisation which occurs, and once again continue to monitor the temperature at regular intervals.
- Wait until the temperature has fallen to about 25–30 °C.
Teaching notes
Once the experiment is over the thermometer can easily be removed by flushing with water, since sodium thiosulfate is water-soluble, or by re-melting the solid. Do not heat the test tube directly over a Bunsen flame as at higher temperatures the thiosulfate decomposes and may form toxic products.
The temperature changes occurring show a steady fall as the liquid cools. When a crystal is added to the supercooled liquid, the temperature rapidly rises as solidification takes place, confirming this process is exothermic. The solid then cools to room temperature.
All solids exhibit supercooling to a greater or lesser extent, but sodium thiosulfate is particularly prone to exhibiting this metastable condition. With more time available, it is possible to cool down the melt to a value well below room temperature, but to achieve this involves waiting for more time to elapse, thus lengthening the experiment considerably.
An impressive teacher demonstration can be carried out using cold running water to cool the melt down rapidly to about 5–10 °C before a crystal is added to seed the crystallisation process. The test tube warms up extremely rapidly and becomes quite hot in the process.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet