When solutions of two soluble salts are mixed, a solid may form. The solid is called a precipitate, and the reaction is called a precipitation reaction. Precipitation reactions are used to make insoluble salts
In this experiment the soluble salts are magnesium sulfate and sodium carbonate, and the insoluble salt formed is magnesium carbonate, which can be filtered, dried and collected.
This is a short standard class experiment. It should take no more than 20 minutes to the point at which the wet product can be set aside to dry.
If the solutions can be provided in pre-measured 25 cm3 quantities in labelled containers, distribution of chemicals and control of quantities can be easily managed, and the practical work can begin without delay.
Sodium carbonate in dilute solution is weakly alkaline. So the few other safety issues are essentially restricted to safe handling of glassware. Even these can be minimised by the use of polythene filter funnels. This experiment is therefore suitable as a class experiment for most classes.
Equipment
Apparatus
- Eye protection
- Conical flasks (100 cm3) x2
- Filter funnel (65 mm diameter or similar, note 1)
- Filter papers (size suited to funnels used)
Apparatus notes
- Polythene filter funnels are safer and cheaper than glass funnels. The size of filter paper, when folded, should match the funnel size. The cheapest grade of filter paper is okay for this experiment.
Chemicals
- Sodium carbonate solution, 0.5 M, 25 cm3
- Magnesium sulfate solution, 0.5 M, 25 cm3
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection.
- If the reagent solutions can be distributed in pre-measured quantities, waste is reduced and lesson organisation is easier. All containers used for these solutions should be labelled.
- Sodium carbonate solution, Na2CO3(aq) – see CLEAPSS Hazcard HC095a and CLEAPSS Recipe Book RB080.
- Magnesium sulfate solution, MgSO4(aq) – see CLEAPSS Hazcard HC059b.
- Magnesium carbonate, 3MgCO3.Mg(OH)2.3H2O(s) – see CLEAPSS Hazcard HC059b.
Procedure
- Mix 25 cm3 of magnesium sulfate solution and 25 cm3 of sodium carbonate solution in a conical flask.
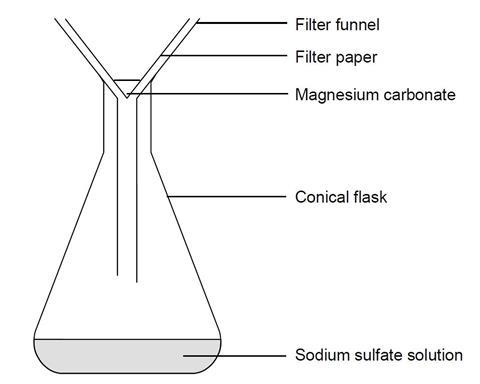
- Place the filter funnel in the neck of another conical flask.
- Fold the filter paper to fit the filter funnel, and put it in place.
- Swirl the reaction mixture gently, and pour a little at a time into the filter paper in the funnel. Only pour in enough solution at a time to leave the solution level 1 cm below the rim of the filter paper. Allow to filter through.
- A clear solution should collect in the flask. If the solution is not clear, and white cloudiness remains in it, you will need to repeat the filtration.
- Remove the wet filter paper carefully from the funnel and place on a clean dry paper towel. Label with your name(s) and leave in a warm place, safe from interference, until it has dried completely (a few hours).
Teaching notes
There are no significant hazards in this experiment, except for the risk of broken glass if a flask is knocked over.
The formation of precipitates on mixing two solutions is met frequently in chemistry. This experiment is intended as a first introduction to this phenomenon for 11–14 year olds, as well as to practical filtration techniques. The experiment can be made more exciting visually by making a coloured salt such as copper(II) carbonate; in this case the chemical hazard level is slightly higher, since copper(II) carbonate is HARMFUL.
Because this is intended as a first introduction, the interpretation should be restricted to developing the word equation as a summary of what has happened:
magnesium sulfate + sodium carbonate → magnesium carbonate + sodium sulfate
Suggesting the name of the salt left in solution is not easy for students at this stage. It needs to be approached carefully, probably by group or whole class discussion. Using cut-out card labels: ‘sodium’, ‘magnesium’, ‘carbonate’ and ‘sulfate’ for students to move around will help many of them grasp the idea of ‘swapping partners’.
You could add some interest to which salts are used, and which salts are formed. Mention their uses, if this helps the class to see that these substances are not just important in the laboratory. See below.
Background information
Magnesium sulfate is known as Epsom salts. This is because the water found at the spa at Epsom in Surrey contains this salt in quite high concentration. Epsom salts are rarely used nowadays, but were used in medicine as a purgative.
Sodium carbonate is found naturally in high concentrations in the soda lakes of Kenya and Tanzania in East Africa. It is also manufactured in vast quantities and used in many different industries, including the chemical industry itself and in glass making. It is found in the home as washing soda, and in some detergent powders. For more about sodium carbonate in general, you may be interest in the Royal Society of Chemistry Book, Sodium carbonate: a versatile material.
Magnesium carbonate is found in the mineral dolomite, mixed with calcium carbonate. Most limestones contains a proportion of magnesium carbonate – some a very high proportion. Magnesium carbonate is used in industry as a major source of magnesium compounds, it is used in many medical preparations to treat indigestion and it is also used as gym chalk.
Sodium sulfate, known as Glauber’s salt, is found (like Epsom salts) in some natural brines. It is used in large quantities in industries such as wood pulp production, glass-making, and detergents, and is also as a mild laxative.
If this experiment is used with older students, you can ask them to work out the symbol equation:
MgSO4(aq) + Na2CO3(aq) → MgCO3(s) + Na2SO4(aq)
The ionic equation, together with the concept of ‘spectator ions’, is likely to be appropriate for fewer students. However, this is not likely to be the experiment where the concept of spectator ions is introduced, as there are better examples, with visual colour clues to what is happening. The ionic equation is:
Mg2+(aq) + CO32-(aq) → MgCO3(s)
and the spectator ions are: Na+(aq) and SO42-(aq)
Student questions
Here are some possible questions for students.
- What did you see happen in the flask when the solutions mixed?
- What has collected in the filter paper? Describe what you can see.
- What is the name of the solid salt you have made?
- Suggest the name of the salt left in solution in the flask at the end. Explain how you decided on this name.
- Complete the word equation for this reaction: magnesium sulfate + sodium carbonate → … + …
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet