In this experiment the water of crystallisation is removed from hydrated blue copper(II) sulfate. After cooling the anhydrous copper(II) sulfate formed is then rehydrated with the same water
Students remove the water of crystallisation from hydrated copper(II) sulfate by heating. Condensing the vapour produced in a second test tube collects the water. The white anhydrous copper(II) sulfate is then rehydrated and the blue colour returns.
This experiment can be carried out in pairs by students. It should take no more than 30–40 minutes.
Equipment
Apparatus
- Eye protection
- Test tubes x2
- Delivery tube (right-angled)
- Beaker, 250 cm3
- Bunsen burner
- Clamp and stand
Chemicals
- Copper(II) sulfate(VI)–5–water (powdered), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), about 5 g
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection.
- Copper(II) sulfate(VI)-5-water, CuSO4.5H2O(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC027c.
Procedure
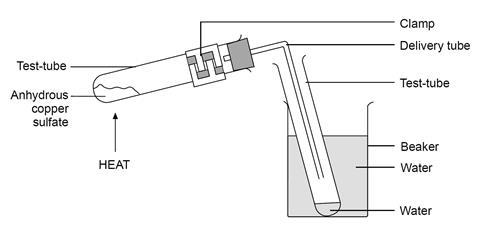
- Set up the apparatus as shown (but without water in the receiving tube – this is to be collected during the experiment), placing about 5 g of powdered hydrated copper(II) sulfate in the test tube. Make sure that the tube is clamped near the bung as shown.
- Heat the blue copper(II) sulfate until it has turned white. Move the flame along the length of the test tube from time to time (avoiding the clamp) to prevent water condensing on the cooler regions and then running down on to the hot solid, possibly cracking the test tube. Do not heat too strongly, nor allow the white colour to darken, as the copper sulfate may decompose to produce toxic sulfur oxides.
- Act quickly to prevent suck-back if the level of water collecting in the test-tube reaches the end of the delivery tube. Lift the clamp stand so that the delivery tube does not reach into the water in the test tube.
- Allow the anhydrous copper(II) sulfate to cool back to room temperature.
- Holding the test tube containing anhydrous copper(II) sulfate in one hand, pour the collected water very slowly on to the white powder. What observations can you make?
- Record any observations made during the heating process and when the water was poured back onto the anhydrous copper(II) sulfate.
Teaching notes
Ensure that the students have clamped the test-tube at the end nearest the bung before they start the experiment, otherwise they will be heating the clamp as well as the test tube.
Warn about, and watch for, suck-back. Demonstrate how to lift the entire clamp stand and apparatus.
The reaction involved is:
CuSO4.5H2O(s) (pale blue solid) ⇌ CuSO4(s) (“dirty” white solid) + 5H2O(l)
Students should observe the colour change from pale blue to white and the change back to blue when water is added. The colour change on adding water to anhydrous copper(II) sulfate has been used as a test for the presence of water in a liquid.
The more observant should notice that the addition of water to anhydrous copper(II) sulfate is exothermic, as the tube becomes noticeably hot if the water is added very slowly. They should therefore conclude that the same quantity of energy is absorbed when the endothermic thermal decomposition takes place.
Perhaps in subsequent class discussion students could be asked why anhydrous copper(II) sulfate would not be a feasible fuel for the future.
More able and older students might be asked to calculate the enthalpy change occurring during this process. They will need to find out from a data book the standard enthalpies of formation for anhydrous and hydrated copper(II) sulfate, as well as that for water.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















1 Reader's comment