Try this demonstration or class experiment to investigate how varying amounts of fuel and oxygen affect combustion
This practical can be used to introduce the idea that the relative amounts of a fuel and oxygen (from air) are important in combustion, and that there will be an optimum ratio in which the two substances react. This leads on to the idea of chemical equations.
In the case of a class experiment, where students generate the hydrogen themselves under strict supervision, all the hydrogen generators must be collected once the test tubes have been filled and before any flames are lit, to prevent the possibility of accidental or deliberate ignition of the hydrogen in the generator. This has caused a number of accidents in the past. Alternatively the test tubes could be filled with hydrogen beforehand, or by students under supervision, from a steady cylinder supply.
The time for carrying out the demonstration should be about five minutes. More time (20–30 minutes) will be needed for the class experiment.
Equipment
Apparatus
For one demonstration
- Eye protection
- Glass or plastic trough
- Test tubes, x3
- Test tube rack
- Delivery tube for gas collection over water (see the diagram below)
- Rubber bungs to fit test tubes, x3
- Waterproof marker pen
- Wooden splints
- Boss, clamp and stand
For students’ experiments
- Eye protection
- Conical flask, 100 cm3
- One-hole bung to fit flask
- Delivery tube for gas collection over water (see the diagram below)
- Glass or plastic trough for gas collection
- Measuring cylinder, 50 cm3
- Test tubes, x3
- Rubber bungs to fit test tubes, x3
- Test tube rack
- Waterproof marker pen
- Wooden splints
- Boss, clamp and retort stand
- Access to a fume cupboard, for storing hydrogen generators after use
Chemicals
For one demonstration
-
Access to a source of hydrogen gas (EXTREMELY FLAMMABLE): a cylinder and regulator, or chemical generator (see Generating, collecting and testing gases) fitted with a length of rubber tubing, see CLEAPSS Hazcard HC048
For students’ experiments
- Hydrochloric acid, 2 M (IRRITANT), 50 cm3
- Zinc, granulated, 4–5 g
- Copper(II) sulfate solution, about 0.5 M (MINIMAL HAZARD), a few drops
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hydrogen, H2 (g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC048.
- Dilute hydrochloric acid, HCl(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043. The total volume of hydrogen that could be produced using the given quantities (zinc is in excess) is just over 1000 cm3.
- Zinc, Zn(s) – see CLEAPSS Hazcard HC107. The rate at which hydrogen is produced will depend on the surface area of the zinc granules. Avoid large lumps and carry out a test run before the class to check that the volume and rate of hydrogen production is sufficient to fill three test tubes to a marked volume once the air has been flushed out of the apparatus. Adjust the amount of zinc granules and/or acid accordingly.
- Copper(II) sulfate solution, CuSO4 (aq), (MINIMAL HAZARD at concentration used) – see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031. The copper sulfate reacts with the zinc, forming a deposit of copper metal on the zinc. This acts as catalyst, speeding up the production of hydrogen. A single dropping bottle of the solution should be provided at a central point.
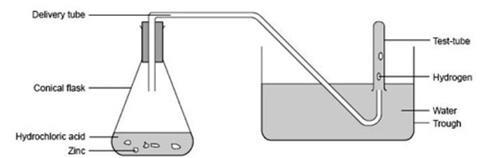
Procedure
Teacher demonstration
- Mark three of the test tubes with a waterproof pen at the quarter, half and three-quarters full mark respectively.
- Fill the trough with water and immerse the test tubes in it so that they fill with water. Stand the rubber bungs on the bottom of the trough so that the test tubes can be pushed downwards on to them when full, to seal them.
- Connect the source of hydrogen to the delivery tube. Clamp a water-filled test tube in position over the trough so that the end is well immersed in the water. Adjust the flow of hydrogen so that a test tube can easily be filled to a given mark. Allow the gas to escape for a minute or two to purge all the air from the system. If the hydrogen contains little or no air, then a test tube full should ignite without a ‘pop’ and burn quietly. (Adding a few centimetres of magnesium ribbon with the zinc gives the gas evolution a rapid start, helping to flush out the air more quickly.)
- Fill each of the marked test tubes to the mark by holding it vertically over the end of the delivery tube. Then move it sideways and raise it slowly until air enters the tube and fills it. Immediately press the filled test tube down onto a bung to seal it and place it in a rack. Repeat with the remaining test tubes.
- Ignite the gas mixture in each of the tubes in turn either by using a lighted splint or by holding each tube upside down before removing the bung and then passing the mouth of the tube briefly through a Bunsen flame. The mixtures should ignite with explosive ‘pops’ of varying intensity.
Student experiments
- Mark three of the test tubes with a waterproof pen at the quarter, half and three-quarters full mark respectively.
- Fill the trough with water and immerse the test tubes in it so that they fill with water. Stand the rubber bungs on the bottom of the trough so that the test tubes can be pushed downwards on to them when full to seal them.
- Place the zinc granules in the flask, loosely fit the stopper carrying the delivery tube and clamp the flask so that the end of the delivery tube is well below the surface of the water in the trough.
- Measure out 50 cm3 of the dilute hydrochloric acid supplied, remove the delivery tube, and add the acid to the zinc granules in the flask. Add a few (5–10) drops of copper sulfate solution. Swirl to mix and reconnect the delivery tube.
- Allow the flow of hydrogen bubbles from the delivery tube to escape for a minute or two to expel all the air from the flask.
- Fill one of the water-filled test tubes to the mark with hydrogen by holding it vertically, with its mouth under water, over the end of the delivery tube. Then move it sideways and raise it slowly until air enters the tube and fills it. Immediately press the filled test tube down on to a submerged bung to seal it and place it in the rack. Repeat with the other two test tubes.
Important
Before proceeding to the next step, remove all the hydrogen generating apparatus to a safe place.
- Ignite the gas mixture in each of the tubes in turn either using a lighted splint or by holding each tube upside down before removing the bung and then passing the mouth of the tube briefly through a Bunsen flame. The mixtures should ignite with explosive ‘pops’ of varying intensity.
Teaching notes
The reaction occurring is the combustion of hydrogen to form water:
2H2 (g) + O2 (g) → 2H2 O(g), ΔH = –484 kJ mol–1
The energy released appears as heat, light, sound and kinetic energy, similar to the situation in an internal combustion engine. Mixtures of air and flammable gases usually have quite narrow explosive limits but hydrogen–air mixtures are explosive over a much broader range (4–77 mol% hydrogen).
The best ‘pop’ is usually achieved with a mixture containing 20–40 % by volume of hydrogen. Everyday examples of the need for a sufficient supply of air for effective combustion, eg Bunsen burner, household gas appliances and internal combustion engines, can be pointed out.
Given the formula for water as H2O, the ideal volume ratio of hydrogen and oxygen for complete combustion could be considered by the class – taking into account Avogadro’s Law. Using this ratio and the volume composition of air, the volume of air necessary for complete combustion of the hydrogen can be worked out and compared with class results.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet