Six solutions are provided, a mystery to learners
They are unnamed but are known to be bromine water, iodine solution, iron(II) sulphate, potassium dichromate, silver nitrate and sodium sulphite.
Introduction
This is an ill-defined problem in that the ‘best method’ has not been defined. Each student group has to find the best balance between the method that uses fewest additional chemicals or test papers, and the one that takes the shortest time. There is no one correct answer.
Teachers who have not used the problems before should read the section Using the problems before starting.
Prior knowledge
Familiarity with redox reactions, the ability to apply the electrochemical series (standard electrode potentials, Eo list), associated colour changes such as dichromate changing from orange to green when it is reduced in the presence of H+(aq) and tests for ions. A detailed knowledge is unnecessary as students are encouraged to consult textbooks and data books during the exercise.
Resources
Data books and inorganic textbooks should be available for reference.
Unnamed, but numbered bottles containing solutions of bromine water, iodine solution, iron(II) sulphate,1 potassium dichromate, silver nitrate and sodium sulphite should be provided at the start of the exercise.
The precise concentrations are unimportant – laboratory reagents can be used where available, otherwise approximately 5 g solid per 100 cm3 of solution. The iodine solution and potassium dichromate should be made up so that their colours roughly match that of the bromine water.
Students can request apparatus and chemicals during the practical session, and these should be issued if they are safe to use. In particular, flame test equipment will probably be required, but it should not be on view.
Special safety requirements
There are hazards if the solutions are heated; and the staining effect of silver nitrate and iodine solutions should be noted.
Possible methods
1. The colourless solutions are the iron(II) sulphate, silver nitrate and sodium sulphite. Add each of these in turn to the three coloured solutions.
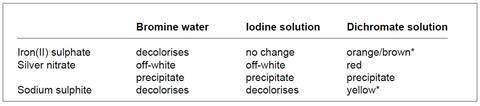
* green in the presence of dilute acid
a. The colourless solutions are identified as follows:
- silver nitrate – precipitate each time, in particular the off-white precipitates;
- sodium sulphite – decolorises two of the three coloured solutions; and
- iron(II) sulphate – decolorises one of the three coloured solutions.
b. The coloured solutions are identified from the results table.
- To confirm the identities of the halogens, starch solution (blue/black colour with iodine) or an organic solvent (colour of lower layer) could be used.
2. Perform standard ion tests such as a–e below.
a. Flame tests (sodium and potassium).
b. Adding metals to the solutions, such as:
- copper, displacing silver from silver nitrate; and
- zinc or magnesium, displacing silver from silver nitrate and iron from iron(II) sulphate.
c. Electrolysis identifies the silver nitrate because black (finely divided) silver is produced at the cathode.
d. Standard tests for ions, such as:
- hexacyanoferrate(III) for Fe2+;
- a chloride solution for silver nitrate;
- brown ring test for nitrate;
- barium nitrate solution for sulphate and sulphite; and
- warming sodium sulphite solution with dilute hydrochloric acid to give sulphur dioxide (this may not work in this case because the solutions are too dilute).
e. In principle, the solutions could be evaporated to dryness, with further heating of any residual solids in a fume cupboard. This is a time consuming method and the solutions are so dilute that only small quantities of solids would become available. Thus the method is not
recommended although reactions will occur:
- bromine water leaves no solid;
- iodine leaves a solid (potassium iodide and iodine) which gives purple fumes of iodine;
- silver nitrate gives a white solid which decomposes to give brown fumes of nitrogen dioxide as well as oxygen; and
potassium dichromate gives oxygen and a greenish solid. The other two samples do not break down in a recognisable way.
Downloads
Six solutions creative problem solving
PDF, Size 0.2 mb
Additional information
This resource is part of our Creative problem-solving in chemistry collection.


















No comments yet