Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. This can be reversed using electrolysis to decompose the compound
This experiment involves the synthesis of a metal salt by direct reaction of a metal and a non-metal. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent.
Zn + I2 → ZnI2
The experiment can be extended to show the decomposition of a compound into its elements.
This experiment can be used to illustrate the differences between metallic and non-metallic elements and their reaction to form a compound – a metal salt – with new properties.
The reaction can be easily reversed using electrolysis to decompose the compound back into its elements. These are easily recognisable from their distinctive appearances.
Both parts of the experiment can be done either as demonstrations or as class experiments. Each part should take about 10 mins as a demonstration; longer as a class experiment.
Equipment
Apparatus
- Eye protection
Each group (or demonstration) requires:
- Test tubes (100 x 16 mm) x3
- Test tube bung
- Test tube rack
- Measuring cylinder (10 cm3)
- Small filter funnel
- Filter paper
- Teat pipette
- Thermometer (0–100 °C)
- Spatula
- Watch glass
- Weighing boat or suitable container for zinc powder
For the extension work:
- Beaker (100 cm3)
- Pair of graphite electrodes mounted in a rubber bung
- Electrical leads and crocodile clips
- Source of 3–6 V DC, either battery or power supply
- Torch bulb in a suitable holder
- Spatula
Chemicals
Chemicals are for one demonstration or one group of students:
- Iodine (HARMFUL), about 0.5 g (note 1)
- Zinc powder (HIGHLY FLAMMABLE), about 0.5 g (note 2)
- Ethanol (HIGHLY FLAMMABLE) or IDA (Industrial Denatured Alcohol) (HIGHLY FLAMMABLE, HARMFUL) about 5 cm3
For the extension work:
- Distilled water, about 20 cm3
- Dilute hydrochloric acid, 1 M or sulfuric acid, 1 M (IRRITANT), about 20 cm3
Chemical notes
- The solid iodine should be powdered by grinding in a mortar in a fume cupboard. For a class experiment a stoppered test tube containing 0.5 g of powdered iodine should be supplied to each group of students.
- For a class experiment each group of students should be supplied with a pre-weighed sample of 0.5 g zinc powder in a weighing boat or a test tube.
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection.
- Iodine, I2(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC054.
- Zinc powder, Zn(s), (HIGHLY FLAMMABLE, DANGEROUS FOR THE ENVIRONMENT)– see CLEAPSS Hazcard HC107.
- Ethanol, C2H5OH(l), (HIGHLY FLAMMABLE or HIGHLY FLAMMABLE and HARMFUL if using IDA) – see CLEAPSS Hazcard HC040a.
- Zinc iodide, ZnI2(s), (IRRITANT) – see CLEAPSS Hazcard HC054.
- Dilute hydrochloric acid, HCl(aq) or dilute sulfuric acid, H2SO4(aq) (IRRITANT) – see CLEAPSS Hazcard HC047a.
Procedure
Synthesis of zinc iodide
- Measure out 5 cm3 of ethanol using a measuring cylinder. Place a thermometer in the ethanol and record the temperature.
- Add the ethanol to 0.5 g of powdered iodine in a test tube. Stir carefully, using the thermometer, to dissolve the iodine. The solution should be dark brown. Note the temperature.
- When all the iodine has dissolved, slowly add the zinc powder using a spatula and stir the mixture with the thermometer. The temperature should rise, indicating an exothermic reaction. When the reaction is finished, the colour of the iodine should have faded and excess zinc will be left. If not, add further small amounts of zinc powder and stir until the brown colour due to iodine has gone.
- Filter the solution into another test tube. Using a teat pipette, transfer a few drops of the filtrate on to a watchglass and allow the solvent to evaporate. This can be speeded up by placing the watchglass on a beaker containing some hot water. Zinc iodide will be left as a white solid.
Decomposition
- Pour the remainder of the solution containing the zinc iodide into a 100 cm3 beaker. Add about 20 cm3 of distilled water and stir to mix.
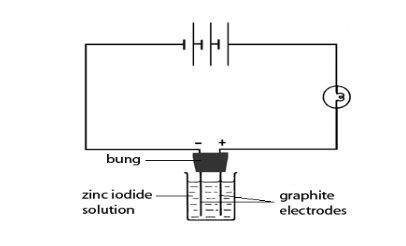
- Clamp the bung carrying the two graphite electrodes over the beaker, so that the bottoms of the electrodes are immersed as far as possible in the solution. It may be easier just to rest the bung in the beaker so that the electrodes touch the bottom.
- Using the leads and crocodile clips, connect the electrodes and the bulb in series and then to the power supply as shown in the diagram on this page. The bulb should glow to show that the circuit is complete, and that electrolysis is occurring.
- If the bulb does not glow, raise the bung out of the solution and check the connections by touching both electrodes at once with a metal spatula. If the bulb lights up, put the electrodes back into the solution. If there is still no indication of electrolysis, add a small amount of zinc iodide from the watchglass to the solution and stir. Repeat until the bulb starts to glow.
- Allow electrolysis to continue for a few minutes. Note any changes occurring around the electrodes in the solution – a brown colour (due to iodine) should develop in the solution around the positive electrode. There may be some effervescence at the negative electrode.
- Disconnect the power supply. Lift the electrodes out of the solution. Wash them under a tap. The bottom of the negative electrode should be covered with a silver-grey layer of zinc metal.
- The zinc deposit can be tested (and removed) by immersing the tip of the electrode in a little dilute acid. It reacts and dissolves, giving off a colourless gas (hydrogen).
Teaching notes
This reaction shows the synthesis of a compound from two elements, each with their own distinctive appearance and properties. A practical worksheet could involve drawing up a table of properties (type of element, appearance, and so on) for each of the elements and the compound formed.
The reaction can also be used to illustrate the direct reaction of a typical metal and non-metal. It is one of the few reactions of the halogens (Group 17) with a metal that students can do safely themselves.
A useful extension of this experiment is the decomposition, by electrolysis, of the compound formed back into its elements.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet