Students weigh magnesium and heat it in a crucible, noting the change in weight they can discover the formula for magnesium oxide
Video support and linked resources
This experiment is included in our Conservation of mass video, along with supporting resources, including illustrated technician notes, integrated instructions, worksheets, a structure strip and more.
The practical activity takes around 30–45 minutes, depending on the competence of the class. Students should all be standing and should wear eye protection. Students with long hair should tie it back.
It is a good idea for students to practice lifting the lid on and off the crucible and the crucible off the pipe clay triangle before they start. This has the added bonus of checking that all the tongs are functioning correctly.
To enable students to light their Bunsen burners they will need access to matches or lighters. Alternatively, light one or two Bunsen’s around the room and students can light their own using a splint.
The most significant hazard in this experiment is the hot apparatus. Warn students that it will take some time to cool down.
For classes with shorter attention spans, the final step of heating to constant mass could be omitted.
Equipment
Apparatus
- Eye protection.
- Access to a balance (2 decimal places)
- Per pair or group of students:
- Crucible with lid
- Tongs
- Pipe clay triangle
- Bunsen burner
- Tripod
- Heat resistant mat
- Emery paper (optional)
Chemicals
- Magnesium ribbon, about 10–15 cm
Health, safety and technical notes
- Read our standard health and safety guidance
- Wear eye protection.
- Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059a. Fresh, clean magnesium is best for this experiment. If the magnesium is tarnished then emery or sand paper will be required to clean it.
Procedure
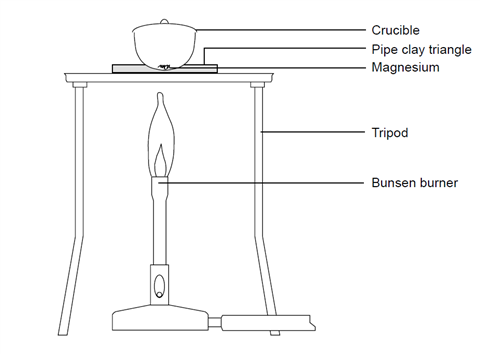
- Cut a piece of magnesium about 10–15 cm long. If it is looking tarnished or black then clean it using the emery paper. Twist it into a loose coil.
- Weigh the crucible with the lid (mass 1) and then the magnesium inside the crucible with the lid (mass 2).
- Set up the Bunsen burner on the heat resistant mat with the tripod. Place the pipe clay triangle over the tripod in a Star of David formation, ensuring that it is secure. Place the crucible containing the magnesium in the pipe clay triangle and put the lid on.
- Light the Bunsen burner and begin to heat the crucible. It is best to start with a gentle blue flame, but you will need to use a roaring flame (with the air hole fully open) to get the reaction to go.
- Once the crucible is hot, gently lift the lid with the tongs a little to allow some oxygen to get in. You may see the magnesium begin to flare up. If the lid is off for too long then the magnesium oxide product will begin to escape. Don’t let this happen.
- Keep heating and lifting the lid until you see no further reaction. At this point, remove the lid and heat for another couple of minutes. Replace the lid if it appears that you are losing some product.
- Turn off the Bunsen burner and allow the apparatus to cool.
- Re-weigh the crucible with lid containing the product (mass 3).
- Heat the crucible again for a couple of minutes and once again allow to cool. Repeat this step until the mass readings are consistent. This is known as heating to constant mass.
Teaching notes
Students should have recorded the following masses:
- mass 1 = crucible + lid
- mass 2 = crucible + lid + magnesium
- mass 3 = crucible + lid + product
This should allow them to calculate the mass of the mass of the magnesium (mass 2 – mass 1) and the mass of the product (mass 3 – mass 1). They could also calculate the increase in mass (mass 3 – mass 2), which corresponds to the mass of oxygen.
The equation is:
- Magnesium + oxygen → magnesium oxide
- 2Mg + O2 → 2MgO
Students sometimes get unconvincing results to this experiment. It is worth evaluating what they have done as there are several reasons why their results may be disappointing:
- the magnesium oxide product may escape as they lift the lid
- not all the magnesium may have reacted (the product may still look a bit grey rather than white)
- they may have prodded the product with their splint so not all of it got weighed (more common than you might expect)
- not taring the balance correctly when measuring the mass
- having the magnesium coiled too tightly so that not all of it reacts
Finding the formula of magnesium oxide
Method one
- To find the formula of magnesium oxide, students will need the mass of the magnesium and the mass of the oxygen. They will also require the relative atomic masses. Magnesium is 24 and oxygen is 16.
- They should divide mass by the atomic mass for each element. The gives the number of moles of each.
- Having done this for both elements, they should find the ratio between the two by dividing them both by the smallest number.
- The ratio should be close to 1:1 as the formula of magnesium oxide is MgO.
- Example calculation:
- Mass magnesium = 2.39 g
- Mass magnesium oxide = 3.78 g
- So mass oxygen = 1.39 g
- Number moles Mg = 2.39/24 = 0.0995
- Number moles O = 1.39/16 = 0.0868
- Divide by the smallest to give the ratio aproximately 1 Mg : 1 O
- This would suggest a formula of MgO, which is the correct formula
Method two
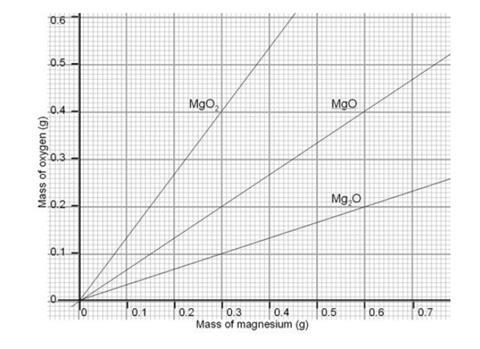
- Students will need the mass of the magnesium and the mass of oxygen which has combined with it. You will need a copy of the graph for the class.
- All students plot their masses of magnesium and oxygen onto the graph. The majority of the class’ results should fall on or near the line representing the formula MgO, a 1:1 ratio. This helps to show clearly any anomolous results and should help to convince students who are disappointed by a 1:1.25 ratio, for instance, that the correct formula really is MgO.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















4 readers' comments