Show how the energy of a chemical reaction can be given out as light by revealing how a solution of sodium chlorate(I) oxidises an aqueous solution of luminol (3-aminophthalhydrazide) to produce a blue chemiluminescent glow – without any increase in temperature.
A solution of sodium chlorate(I) oxidises an aqueous solution of luminol (3-aminophthalhydrazide). The reaction gives out a blue chemiluminescent glow without any increase in temperature of the mixture.
Lesson organisation
This demonstration experiment shows that a chemical reaction can give out energy as light instead of heating up its surroundings. The demonstration can also be used to stimulate interest in chemistry at an Open Day or other public event.
Equipment
Apparatus
- Eye protection
- Conical flasks with stoppers (1 dm3), 2
- Beaker (2 dm3)
- Thermometer (0 – 100 °C)
- Balance (1 d.p.)
Chemicals
- Household bleach 100 cm3 OR sodium chlorate(I) solution (up to 14%) (CORROSIVE / IRRITANT) (Notes 1, 2 and 3)
- Luminol (3-aminophthalhydrazide), 0.4 g (IRRITANT) (Note 4)
- Sodium hydroxide pellets, 4.0 g (CORROSIVE)
- Fluorescein (or sodium fluorescein) powder (optional)
Health, safety and technical notes
- Read our standard health & safety guidance
- Wear eye protection. Goggles should be worn when preparing the solution.
- Luminol, C8H7N3O2(s), (IRRITANT) - see CLEAPSS Hazcard HC004b and CLEAPSS Recipe Book.
- Household bleach, NaOCl(aq), (IRRITANT) - see CLEAPSS Hazcard HC089.
- Sodium chlorate(I) solution, NaOCl(aq), (CORROSIVE) - see CLEAPSS Hazcard HC089.
- Sodium hydroxide pellets, NaOH(s), (CORROSIVE) - see CLEAPSS Hazcard HC0091a.
- Fluorescein - see CLEAPSS Hazcard HC032.
Technical notes
- Household bleach, which is typically a 5% ‘available chlorine’ solution, can be used. Make sure that the household bleach contains sodium chlorate(I) (sodium hypochlorite), NaOCl, and not hydrogen peroxide, as the bleaching agent. Many household bleaches nowadays also contain thickeners and/or detergents. Use ‘economy’ bleaches without any additives.
- The sodium chlorate(I) solution sold by chemical suppliers contains up to 14% available chlorine. It has a limited shelf life. Adjust the volumes of bleach and water to make up the diluted bleach solution for the demonstration. See Procedure step a.
- Tap water can be used for making up the solutions. The separate solutions are stable for over 12 hours and so can be made up well in advance.
- The luminol does not always appear to dissolve completely, leaving a fine, greenish suspension.
Procedure
Before the demonstration
- Add 100 cm3 of the household bleach solution to 900 cm3 of water in one of the flasks, mix well and stopper. Alternatively, add 50 cm3 of commercial NaOCl solution to 950 cm3 of water. See notes 1 to 3 above.
- In the other flask put 0.4 g of luminol, 1 dm3 of water and 4.0 g of sodium hydroxide. Swirl to dissolve the chemicals and then stopper the flask. See notes 3 and 4 above.
The demonstration
- Take the temperature of the solutions.
- Lower the room lights and slowly pour the two solutions at the same rate into the beaker so that they mix. A pale blue glow will be seen which will last for a few seconds.
- Take the temperature of the mixture. It will be the same as that of the starting solutions.
Teaching notes
For more dramatic effect, pour the solutions into a funnel attached to clear tubing bent into a variety of shapes, such as a spiral.
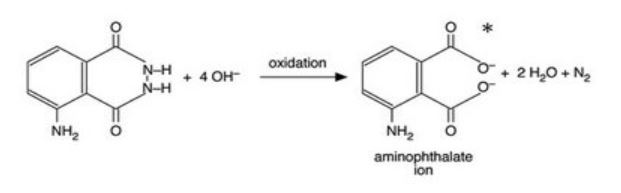
In the reaction, luminol is oxidised by the bleach to the aminophthalate ion, which is produced in an electronic excited state. This gives out energy as light (fluorescence) when it decays to the ground state.
Adding a small amount of fluorescein to the luminol solution, just before the demonstration, will alter the glow to a yellow-green colour.
Chemiluminescent ‘light sticks’ will be familiar to many students. A different reaction is used, involving the oxidation of a di-ester by hydrogen peroxide in an organic solvent. This reaction is much slower, the glow continuing for some hours. One solution is contained inside a vial inside a plastic tube containing the other solution. Bending the plastic tube breaks the vial, allowing the two reactants to mix. Colour effects are obtained by adding dyes to which the excitation energy is transferred, the excited dye molecules then emit light of different wavelengths. It is possible to slow the reaction down by placing the light stick in a freezer.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry. Page last updated July 2016. Health & Safety checked, July 2016


















No comments yet