Try this demonstration to show students how an ionic salt will conduct electricity when molten but not when solid
In this practical, students observe the electrolysis of molten zinc chloride in a fume cupboard, after noting that the salt does not conduct electricity when solid.
The demonstration uses zinc chloride, as this will melt at Bunsen burner temperatures. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Lead bromide decomposes to its elements just by heating without the need for electricity. The electrolysis of lead bromide must be carried out in a fume cupboard.
Like the electrolysis of lead bromide, the electrolysis of zinc chloride should be carried out in a fume cupboard. The chlorine produced at the positive electrode is TOXIC and DANGEROUS FOR THE ENVIRONMENT.
There are quite long periods of waiting, including at least 15 minutes for the electrolysis to take place so if you have access to a webcam, or video camera and a data projector, this would enable students to see what is going on inside the crucible.
If not, bring students up in groups of two or three to view the experiment. They should note at which electrode bubbles are forming but must avoid smelling the bleachy smell (be aware that many students are asthmatic). They should be able to see crystals of zinc around the negative electrode.
It is worth having another, related activity for the class to be getting on with.
Equipment
Apparatus
- Eye protection
- Fume cupboard
- Low voltage (0–12 V) powerpack and electrical leads
- Graphite electrodes, x2, supported in an electrode holder or bung
- Ammeter and/or bulb (in holder)
- Circuit tester (optional)
- Bunsen burner, tripod and heat resistant mat
- Pipeclay triangle
- Crucible
- Clamp and stand
- Metal spatula
- Tongs
- Plastic beaker
- Filter paper and funnel
- Indicator paper and/or starch-iodide paper
Chemicals
- Solid zinc chloride (CORROSIVE, DANGER TO THE ENVIRONMENT)
- Distilled water
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Work in a fume cupboard.
- Zinc chloride, ZnCl2 (s), (CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC108a.
- Chlorine, Cl2 (g), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC022a. Chlorine is a product of the electrolysis.
Procedure
Setting up the electrolysis
- Set up a heat resistant mat, tripod, Bunsen burner and pipeclay triangle. Put the crucible onto the pipeclay triangle, ensuring that it is sitting firmly and is in no danger of falling through.
- Set up the electric circuit with the power pack, ammeter and/or bulb and electrodes in series. Short the circuit at the electrodes with a key or the metal spatula. This is to satisfy yourself and the students that it is working.
- Clamp the electrodes so that they almost touch the bottom of the crucible but do not touch each other. Your set-up should resemble diagram below.
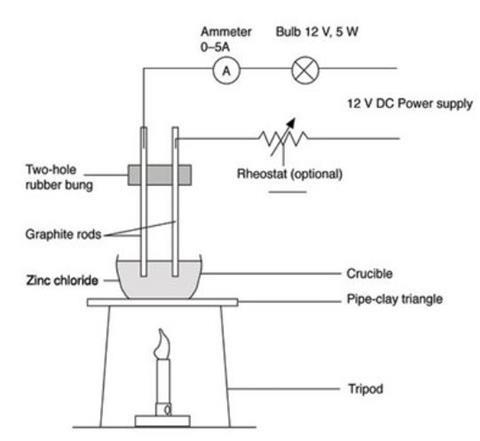
- Fill the crucible to within about 5 mm of the top with the powdered zinc chloride. As it melts the solid will shrink in volume as air escapes and it is important that the level of the molten salt does not drop below the level of the bottom of the electrodes. Ensure that the leads are well out of the way of the Bunsen flame. Using long electrodes can help with this.
Showing that the solid zinc chloride does not conduct electricity
- Begin to heat the crucible with a low to medium Bunsen flame. Watch the leads, and the bung if you are using one, to ensure that you are not overheating them.
- The zinc chloride takes about 3 or 4 minutes to melt. It may be tempting to use a roaring Bunsen flame to speed up the melting, but if you do so the zinc chloride can form a crust over the top. This will prevent students from seeing what is going on, and the liquid salt may boil.
- As the salt melts, the bulb will light up and/or the ammeter will give a reading. Turn the Bunsen down a bit at this point. There will be some heating effect from the electric current which may be enough on its own to keep the zinc chloride molten (as in the industrial electrolysis of aluminium oxide.)
- Bubbles of gas will be seen at the positive electrode. The gas can be confirmed as chlorine by holding moist indicator paper close to the bubbles - it will go red and the edges may start to bleach. A more convincing test is to use moist starch iodide paper which will go black. It is also possible to see crystals of zinc forming on the negative electrode. These can form a bridge across the electrodes, effectively shorting them.
- Electrolyse the molten salt for about 15 minutes, with the current adjusted to about 0.5 A. Check every few minutes that the current remains roughly constant as there is a tendency for it to slowly increase.
- After 15 minutes, turn off the power pack and Bunsen burner and remove the electrodes from the crucible. If this is not done while the salt is still molten the electrodes will stick.
- Leave the crucible to cool for about 10 minutes. You may be able to see zinc crystals on both the electrode and on the surface of the mixture in the crucible. You could stop at this point, but to convince students that a metal really has been made you can separate the zinc from the remaining zinc chloride.
Separating the zinc
- When the crucible is cool to the touch, put it into a beaker of distilled water. (If the water is at all basic like most hard tap waters, the zinc ions will flocculate forming large particles which are far harder to remove from the zinc metal.) The zinc chloride will dissolve (which may take some time) and can be decanted off. Swirl the beaker which will cause the zinc metal to concentrate in the centre of the beaker and decant off most of the liquid.
- Filter the remainder and show students the shiny pieces of metal left on the filter paper. Dry the pieces of metal carefully between further sheets of filter paper and then test with a circuit tester to prove that you have a metallic product. Given that the starting material was zinc chloride and you have made chlorine, most students will have little difficulty in accepting that the metal is zinc.
Teaching notes
If the bulb does not light when testing the circuit, and the electrodes are mounted in a bung, check that the electrodes are not cracked.
The boiling point of zinc chloride is about 730 °C. This can easily be reached by the combination of the heat from the Bunsen burner and the electric current. If the zinc chloride does begin to boil, it can boil over from the crucible and will also produce fumes of zinc chloride in the air. These rapidly turn back to the solid, forming a fine powder.
It is possible to confuse the boiling bubbles with those of chlorine gas being formed. Therefore do not heat the molten zinc chloride too strongly.
Do not try to remove the Bunsen and cool the salt while still electrolysing it, in order to show that the salt only conducts when molten. The heating effect of the electric current will keep the salt molten for several minutes, and when it does cool, a crust forms which is very difficult to remelt.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















3 readers' comments