This activity is designed to elicit common alternative conceptions about the nature of the interactions between an atomic nucleus and electrons
These incorrect ideas tend to be used by students when explaining patterns in ionisation energy
Ionisation energy – true or false?
The statements below refer to this diagram of the electronic structure of an atom.
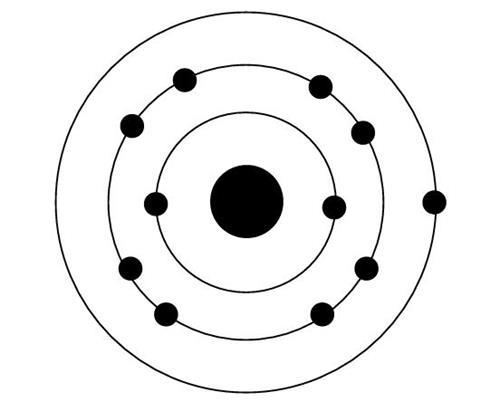
Please read each statement carefully, and decide whether it is correct or not.
- Energy is required to remove an electron from the atom.
- After the atom is ionised, it then requires more energy to remove a second electron because the second electron is nearer the nucleus.
- The atom will spontaneously lose an electron to become stable.
- Only one electron can be removed from the atom, as it then has a stable electronic configuration.
- The nucleus is not attracted to the electrons.
- Each proton in the nucleus attracts one electron.
- After the atom is ionised, it then requires more energy to remove a second electron because the second electron experiences less shielding from the nucleus.
- The nucleus is attracted towards the outermost electron less than it is attracted towards the other electrons.
- After the atom is ionised, it then requires more energy to remove a second electron because the second electron is in a lower energy level.
- After the atom is ionised, it then requires more energy to remove a second electron because it experiences a greater core charge than the first.
- After the atom is ionised, it then requires more energy to remove a second electron because it would be removed from a positive species.
- If the outermost electron is removed from the atom it will not return because there will be a stable electronic configuration.
- The force on an innermost electron from the nucleus is equal to the force on the nucleus from an innermost electron.
- Electrons do not fall into the nucleus as the force attracting the electrons towards the nucleus is balanced by the force repelling the nucleus from the electrons.
- The third ionisation energy is greater than the second as there are less electrons in the shell to share the attraction from the nucleus.
- The force pulling the outermost electron towards the nucleus is greater than the force pulling the nucleus towards the outermost electron.
- After the atom is ionised, it then requires more energy to remove a second electron because once the first electron is removed the remaining electrons receive an extra share of the attraction from the nucleus.
- The atom would be more stable if it ‘lost’ an electron.
- The eleven protons in the nucleus give rise to a certain amount of attractive force that is available to be shared between the electrons.
- The atom would become stable if it either lost one electron or gained seven electrons.
Answers
- True: This is the ionisation energy.
- True: When two charged particles interact the force they experience depends on the size of their charges, and their separation. The greater the separation the smaller the force.
- False: Work has to be done to remove an electron – ie ionisation energy has to be applied. (This is an endothermic step in the Born-Haber cycle.)
- False: Although the second ionisation energy is considerably larger than the first.
- False: The nucleus is attracted to an electron with the same magnitude (size) force as the electron is attracted toward the nucleus.
- False: All the protons in the nucleus attract all the electrons (and vice versa).
- True: For the outermost electron there are 10 shielding electrons (which repel it, and effectively cancel the attraction due to 10 of the 11 protons in the nucleus), so the core charge is +1, but for the next electron removed there are only 2 shielding electrons so the core charge is +9.
- True: As the nucleus is further from the outermost electron. When two charged particles interact the force they experience depends on the size of their charges, and their separation. The same magnitude (size) force acts on both particles, although the forces are in opposite directions. (The force on the electron is directed toward the nucleus, the force on the nucleus acts towards the electron.)
- True: The outermost electron is a 3s electron, the next to be removed a 2p electron, which is at a lower energy (largely because it is closer to the nucleus, and shielded less and hence attracted more).
- True: The first electron experiences a core charge of (11–10=) +1, the second electron experiences a core charge of (11–2=) +9.
- True: The first electron has to be pulled away from a cation with +1 charge, but the second electron has to be pulled away from a +2 charge, so a greater force has to be overcome.
- False: Unless the electron is attracted somewhere else it will be attracted back to the positive sodium ion.
- True: The force acting on both has the same magnitude.
- False: As the nucleus is attracted to the electrons, not repelled! The electrons do not fall in to the nucleus as quantum rules only allow the electrons to occupy certain ‘positions’ ie orbitals.
- False: Although the ionisation energy is greater, the attraction from the nucleus is not shared. The actual reasons are (i) there is less repulsion from other electrons counteracting the attraction from the nucleus; and (ii), because of (i), the ionic radius decreases so the third electron (to be removed) is closer to the nucleus.
- False: Both experience the same magnitude force.
- False: More energy is required to remove the second electron as it is closer to the nucleus, experiences a larger core charge, and is being removed from a more positive species. However, the removal of the 3s electron does not increase the attraction to the nucleus experienced by the other electrons.
- False: The separated cation and electron are less stable, which is why energy is required to ionise the atom.
- False: the positive charge in the nucleus gives rise a force-field that ‘permeates’ through space. However, there is no force produced unless another charged particle (eg an electron) is present in the field. Each electron will interact with the force field due to the nucleus, without ‘using it up’ in any way. (The electrons will, of course, repel each other which may counteract the effect of the attraction towards the nucleus to some extent.)
- False: Although removal of an electron leaves a stable electronic configuration (2.8, isoelectronic with neon), it requires energy as the isolated (negative) electron and the (positive) cation formed would be attracted back together. Gaining seven electrons would give an octet electronic structure (2.8.8, isoelectronic with argon) – however a seven minus ion (Na7–) would not be stable as the repulsions would far outbalance the attractions. There would be eight electrons in the third shell, all repelling each other, and only attracted to a core charge of +1.
Notes
For the full version of this chapter, see downloads below.
Downloads
Ionisation energy
PDF, Size 0.22 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
 Currently reading
Currently readingIonisation energy
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































No comments yet