Design and build an electrical battery, which will give the highest current. The results might be shocking
The battery is to be constructed using materials only likely to be found in a kitchen.
Your final battery must be ready to be connected to the ammeter used by the judges, at the appropriate time.
This experiment should take 60 minutes (this may be rather short to allow for full investigation).
Equipment
- Eye protection
- Useful ’junk’ items
- A milliammeter (or multimeter) with 2 wires fitted, and crocodile clips at the other ends.
- Additional leads and clips so that a number of cells can be constructed
- Bulb
- Something abrasive such as wire wool or glasspaper to break oxide layers on metal surfaces used as electrodes.
Electrodes
- Carbon electrodes (pencils)
- Metal cutlery
- Off-cuts from copper pipes
- Brass curtain rings
- Aluminium foil
Other
- Bicarbonate of soda
- Sodium chloride
- Washing powder
- Washing soda
- Bath salts
- Vinegar
- Lemonade
- Coca cola
- Bleach
- Couple of lemons
- Milk powder
- Sugar
- Flour
Health, safety and technical notes
- Read our standard health and safety guidance here.
- Wear eye protection should be used for washing powder/soda and bleach, and should be goggles for undiluted bleach solutions.
- Wear clothing protection if desired.
- This is an open-ended problem-solving activity, so the guidance given here is necessarily incomplete.
- Bleach could be hazardous if full strength. Even quite dilute bleach is an irritant if more than 0.15 M NaOCl.
- Chlorine gas may be given off when mixing vinegar and bleach, ensure the laboratory is well-ventilated.
- Diluted bleach solutions are of low hazard, but for anything more than very small quantities of bleach, ‘neutralise’ with iron II salts or sodium thiosulphate and then wash to waste.
Possible approaches
(The instructions are adequate for “high ability” groups but students of lower ability may need ‘clues’ to set them on the right course.) There could be three investigations
- Which two metals are best? (Reinforcing the reactivity series.)
- Which electrolyte?
- Is it necessary to arrange for large area of electrodes or a “pile” effect (cells in series)?
This is a very successful problem and always produces results. It can be taken at many different levels, from pure trial & error, to sophisticated chemical thinking. As set out, it asks for the highest current, which is rather more sophisticated than the greatest voltage, because it raises issues of the internal resistance which is probably suitable for 16+ years who could appreciate the theoretical issues.
14–16-year-olds would get a better start on the basis of voltage. The best electrolyte is bleach, but as this is hazardous it could be omitted with younger students. (With bleach you can get up to 0.5 A, without bleach currents seem to be in the milliamp range.)
Constructing a battery of cells is a worthwhile technique. With a restricted range of (safe) chemicals, some teachers think this could be used with junior school students – they would need to be shown how to use the milliammeter, but after that it would be a matter of trial & error, controlling variables etc.
For cells connected in series: (Assume wires have no resistance.)
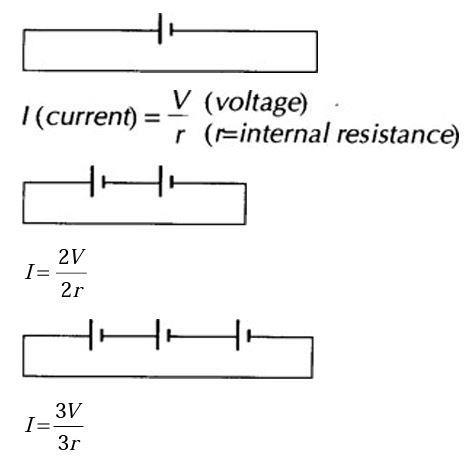
Current stays the same (ie it is no use putting cells in series in an attempt to increase the current).
For cells connected in parallel:
Voltage is same. (Assume wires have no resistance.)
Total resistance (R) of parallel ‘network’
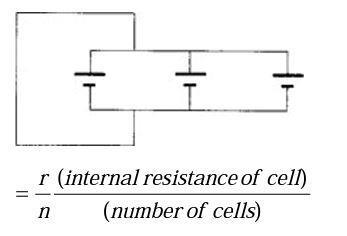
The greater the number of cells, the smaller the resistance of the parallel network, therefore the larger the current.
Notes
In practise, given the limitations of the ‘junk’ apparatus, it is difficult to get a number of cells that are all identical.
Diluted bleach solutions are of low hazard, but for anything more than very small quantities of bleach, ‘neutralise’ with iron II salts or sodium thiosulphate and then wash to waste.
Downloads
Kitchen currents
Experiment | PDF, Size 42.04 kb
Additional information
The resources were originally published in the book In Search of Solution P. Borrows, K. Davies and R. Lewin, Royal Society of Chemistry, 1990.
This experiment was based on an idea contributed by P. Borrows.


















No comments yet