Try this demonstration to investigate the effectiveness of various catalysts for the decomposition of potassium chlorate
In this practical, students observe as potassium chlorate(V) is heated using various oxide catalysts until enough oxygen is produced to light a glowing splint. By noting the time taken for the evolution of oxygen, students can compare the effectiveness of the different catalysts. A water-insoluble catalyst can be separated from the soluble chlorate salt, and weighed to show that it is not used up.
This experiment should only be done as a demonstration, and provides a useful illustration of the effectiveness of different catalysts.
Allow about 30 minutes for the full demonstration, with about 30 minutes needed for the preparation beforehand.
Equipment
Apparatus
- Face shield and safety screens
- Pyrex test tubes, 150 mm x 15 mm, x3 (one for each catalyst to be shown plus one for the control) (see note 7 below)
- Bunsen burner
- Retort stand with boss and clamp
- Filter funnel
- Conical flask (1 dm3) to collect filtrate
- Watch glass, a little larger than the filter paper
- Stopwatch or stopclock
- Top pan balance (2 d.p.)
- Oven
- Wash bottle of deionised water
- Filter paper (eg Whatman no.1)
- Wooden splints
- Mineral wool (optional)
Chemicals
- Potassium chlorate(V) (OXIDISING, HARMFUL), 5 g – this is enough to demonstrate one catalyst plus a control; a further 2.5 g is needed for each additional catalyst
- 0.25 g of each catalyst – the following substances are suitable (see note 8 below):
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT);
- Manganese(IV) oxide (HARMFUL);
- Iron(III) oxide (ferric oxide)
- Silicon dioxide (silica gel)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. The teacher carrying out the demonstration must wear a full face shield and use safety screens to protect the audience and themselves.
- Potassium chlorate(V), KClO3(s) (OXIDISING, HARMFUL) – see CLEAPSS Hazcard HC077.
- Copper(II) oxide, CuO(s) (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026.
- Manganese(IV) oxide, MnO2(s) (HARMFUL) – see CLEAPSS Hazcard HC060.
- Iron(III) oxide, Fe2O3(s) and silicon dioxide, SiO2(s) – see CLEAPSS Hazcards (including HC055A).
- Make sure the test tubes are scrupulously clean as potassium (or sodium) chlorate(V) reacts violently with any oxidisable material. Weigh 2.5 g of potassium chlorate(V) into each of the test tubes. Weigh 0.25 g of copper(II) oxide into the test tube for the catalyst experiment. Mix carefully (do NOT grind the mixtures of potassium chlorate to provide a greater surface area, there is a danger of explosion). If further catalysts are used then add 0.25 g of each to the designated test tube for that catalyst.
- Take great care not to use carbon (charcoal) in place of the catalyst: serious accidents have resulted.
Procedure
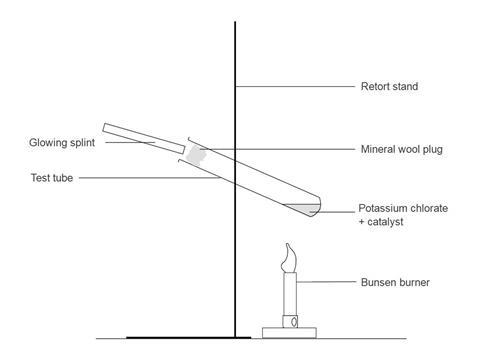
- Set up the apparatus behind safety screens. Clamp the test tube at about 45° with its base about 5 cm above the Bunsen burner. It is best to do a preliminary experiment to find a suitable distance between the Bunsen and the bottom of the test tube. Mark the positions of the clamp and the Bunsen so that it is easy to replace them in the same positions.
- Light the Bunsen, turn the gas fully on and open the air hole, at the same time start the stopwatch. The solid melts and begins to decompose, giving off bubbles of oxygen. Hold a glowing splint at the mouth of the test tube. When it relights, stop the clock and note the time taken for the splint to relight. Turn off the Bunsen.
- Clamp the test tube with potassium chlorate and copper(II) oxide catalyst in the same place as before. Light the Bunsen as before. Start heating, start the stopwatch. Hold a glowing splint at the mouth of the test tube and note the time it takes to relight. This should be between a quarter to one third of the time taken for the original.
- Repeat for any other catalysts if you wish to show further experiments and compare the effectiveness of catalysts.
- Because potassium chlorate(V) is such a powerful oxidising agent you must be careful not to drop the glowing splint into the molten liquid. A loose plug of mineral wool near the mouth of the test tube prevents this.
Catalyst recovery (optional)
- Weigh one of the filter papers after they have been dried in the oven.
- Allow the test tube containing the potassium chlorate and copper oxide to cool. Add a little distilled water to the test tube and warm gently to dissolve the potassium chlorate.
- When it has dissolved, filter the contents of the test tube through the pre-weighed filter paper, using a wash bottle and distilled water to ensure that all the contents are transferred.
- Wash the residue on the filter paper two or three times with distilled water to remove any potassium chlorate. Place the filter paper on a watch glass in an oven to dry.
- When dry (this may need to be the following lesson), reweigh the filter paper and dry catalyst. With care and good technique, the weight of the recovered catalyst is within a few per cent of the initial amount used. This can be tricky as students are often not prepared to accept any loss and the value of the lesson can be diminished.
Teaching notes
Recovering the catalyst is most obvious with the black catalysts, copper(II) oxide and manganese dioxide.
There are opportunities to discuss the factors that must be controlled to make the experiment a ‘fair test’.
You may prefer not to do the catalyst recovery part of the demonstration, leaving it as a ‘thought experiment’.
The overall reaction for the thermal decomposition is:
2KClO3(s) → 2KCl(s) + 3O2(g)
However, it takes place in the following steps:
- Formation of potassium chlorate(VII) (potassium perchlorate), KClO4
4KClO3(s) → KCl(s) + 3KClO4(s) - Decomposition of potassium chlorate(VII)
KClO4(s) → KCl(s) + 2O2(g)
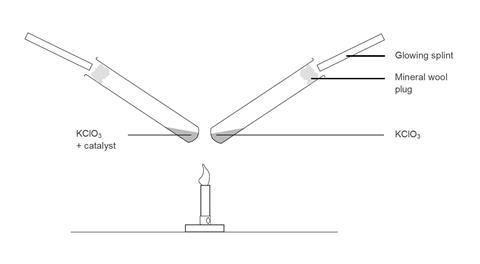
An alternative set-up is to clamp two test tubes above the Bunsen burner so that they are heated equally. The catalyst and control tubes can then be done at the same time, this has a little more impact, but it is more difficult to convince the students that the heating of the two test tubes is equal.
You may prefer to use equimolar quantities of the catalysts rather than equal masses.
Both sodium chlorate(V) and potassium chlorate(V) react at similar rates. There is very little difference in effectiveness between the catalysts.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry

















No comments yet