Use this practical to show that metal cations are preferentially discharged, in relation to the metal’s position in the reactivity series
In this experiment, students electrolyse copper(II) sulfate, iron(II) sulfate and zinc sulfate solutions, before testing for any metal deposited at the electrodes. They then carry out a similar procedure using mixtures of these electrolytes, in order to find out which ion is discharged in preference to the other.
This experiment is best done by students working in groups of two or three.
There is probably insufficient time for each group to do all six electrolyses. It is probably best to assign two metal ions and one mixture to each group, and pool the results from all the groups.
Equipment
Apparatus
- Eye protection (goggles)
- Beaker, 250 cm3
- Beaker, 100 cm3, x2
- Boiling tube
- Test tubes, x2 or 3
- Teat pipette
- Bunsen burner
- Heat resistant mat
- Tripod and gauze
- Platinum electrodes, 1 cm square, with platinum leads sealed through glass tubes, both supported in a rubber bung or cork so that the electrodes are about 2 cm apart, x2 (see note 10 below)
- DC power pack, supplying about 3–4 V
- Light bulb (5V) and holder
- Several lengths of connecting wire, including two fitted with crocodile clips
- Emery paper
Chemicals
- Copper(II) sulfate, about 0.5 M, 200 cm3
- Iron(II) sulfate, about 0.5 M, 200 cm3
- Zinc sulfate, about 0.5 M, 200 cm3 (IRRITANT, DANGEROUS FOR THE ENVIRONMENT)
- Nitric acid, about 4 M (CORROSIVE), 20 cm3
- Aqueous ammonia, about 4 M (IRRITANT), 10 cm3
- (Optional) Solution of mercury(II) chloride (VERY TOXIC) mixed with ammonium thiocyanate (HARMFUL) (see note 11 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection (goggles) throughout.
- Copper(II) sulfate solution, CuSO4(aq) – see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031.
- Iron(II) sulfate solution, FeSO4(aq) – see CLEAPSS Hazcard HC055B and CLEAPSS Recipe Book RB051.
- Zinc sulfate solution, ZnSO4(aq), (IRRITANT, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC108b and CLEAPSS Recipe Book RB106.
- Aqueous nitric acid, HNO3(aq), (CORROSIVE) – see CLEAPSS Hazcard HC067 and CLEAPSS Recipe Book RB061.
- Aqueous ammonia, NH3(aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC006 and CLEAPSS Recipe Book RB006.
- Mercury(II) chloride, HgCl2(s), (VERY TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC062 and CLEAPSS Recipe Book RB057.
- Ammonium thiocyanate, NH4SCN(s), (HARMFUL) – see CLEAPSS Hazcard HC009B and CLEAPSS Recipe Book RB122.
- Platinum electrodes work best – alternatives include graphite.
- If required, to test for zinc in the presence of copper (see teaching notes), the solution containing mercury(II) chloride and ammonium thiocyanate can be prepared by dissolving 2.7 g of mercury(II) chloride and 3 g of ammonium thiocyanate in 100 cm3 of water. The mixed solution at this concentration should be labelled TOXIC.
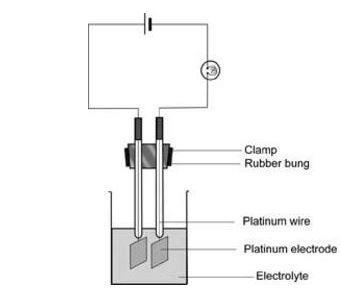
Procedure
- Half-fill the 250 cm3 beaker with water, and heat with a Bunsen burner until boiling. Stop heating and carefully transfer the beaker onto a heat resistant mat.
- Half-fill the 100 cm3 beaker with the copper(II) sulfate solution.
- Clamp the electrodes so that they dip into the copper(II) sulfate solution and connect up the rest of the circuit as shown in the diagram, with the power supply switched off.
- Switch on the current. The bulb should light up. Set the voltage to about 3–4 V, and electrolyse the solution for about 2 min.
- In the meantime, use the teat pipette to transfer no more than 3–4 cm3 of nitric acid into the boiling tube, and then place this in the beaker of hot water.
- Switch off the current and remove the electrodes.
- Place the electrodes under running water, ensuring that all parts that have been in contact with the solution have been washed thoroughly.
- Dip the electrodes one at a time into the hot nitric acid, leaving them in the acid for about 10 seconds. If the electrodes do not reach into the acid or are difficult to remove from the bung, pour the hot acid into a small beaker first.
- Test the solution of nitric acid for the presence of copper ions, using the test described at the end of the procedure.
- Wash the electrodes, the contents of the boiling tube and the 100 cm3 beakers using tap water.
- Repeat steps 2–10 using the iron(II) sulfate solution, and then the zinc sulfate solution, using the tests for iron(II) ions and zinc ions in each case.
- Finally use mixtures of two electrolytes and do the tests for the appropriate metal ions to find out which ion is discharged in preference to the other. Suitable combinations are:
- Copper(II) sulfate and iron(II) sulfate
- Iron(II) sulfate and zinc sulfate
- Copper(II) sulfate and zinc sulfate
Tests for metal ions
Copper ions (Cu2+)
Using the teat pipette, transfer five drops of the solution into a test tube. Wash out the teat pipette and then use it to add the aqueous ammonia drop by drop. Shake the test tube thoroughly throughout the addition. Once all the nitric acid has been neutralised, a pale blue precipitate of copper(II) hydroxide forms initially, but with excess ammonia, this precipitate dissolves and is replaced by a dark blue solution.
Iron(II) ions (Fe2+)
Using the teat pipette, transfer five drops of the solution into a test tube. Wash out the teat pipette and then use it to add the aqueous ammonia drop by drop. Shake the test tube thoroughly throughout the addition. Once all the nitric acid is neutralised, a dirty, dark green precipitate of iron(II) hydroxide eventually forms.
Zinc ions (Zn2+)
Using the teat pipette, transfer five drops of the solution into a test tube. Wash out the teat pipette and then use it to add the aqueous ammonia drop by drop. Shake the test tube thoroughly throughout the addition. Once all the nitric acid is neutralised a white precipitate of zinc hydroxide forms, but this then disappears and is replaced by a colourless solution. If the ammonia is added too quickly the white precipitate will not be seen.
Teaching notes
However tempting it might be, electrolysing solutions of other metal ions gives poor results in practice. This is because the metals concerned tend not to adhere to the platinum cathode very effectively. However, copper, iron and zinc usually work very well.
More support
Use the animations included in our practical video Electrolysis of aqueous solutions to help learners think about which ions are present in an aqueous solution and what is happening at each electrode.
It is essential that the washing process described in step 7 is done thoroughly, otherwise the solution to be tested will contain the original ions, in addition to those derived from the electrolysis.
There are grounds for criticising these tests, as there is the possibility of two metals being deposited on the cathode. For example, during the electrolysis of a mixture of copper ions with zinc ions, if there is any zinc deposited on the cathode in addition to copper, the test described for copper will mask that for zinc. More complex test reactions for zinc ions do exist, producing definitive coloured products, but they would not be understood by the students.
In theory, and indeed in practice, no such mixture of ions should be present, because the experiment is designed to show preferential electrolytic discharge.
However, if you want to know how to test for zinc in the presence of copper, you can use the following technique. Transfer a few drops of the solution to a test tube using a teat pipette. Add a few drops of the mercury(II) chloride-ammonium thiocyanate reagent. A yellow precipitate is formed by copper alone, but if zinc is also present the colour is darker.
Obviously you need to try this out beforehand so that the test can be described and demonstrated with a prior knowledge of the colour changes involved. During the class experiments it may be helpful to have both colours available on display to enable students to compare their results with yours.
Student questions
- For each of the individual solutions being electrolysed, on which electrode (positive or negative) is a metal deposited?
- Write an equation for the electrical discharge of each metal ion: Cu2+, Fe2+, Zn2+.
- When mixtures of electrolytes are used is a mixture of metals deposited at an electrode, or is it a single metal? If so, which?
- What conclusion can you make about the priority of metal ions discharged during electrolysis?
Answers
- Metal ions are discharged at the cathode – the negative electrode.
- The equations are:
- Cu2+(aq) + 2e– → Cu(s)
- Fe2+(aq) + 2e– → Fe(s)
- Zn2+(aq) + 2e–→ Zn(s)
- A single metal only is deposited at the cathode, suggesting that one of the ions is discharged in preference to the other.
- Copper is discharged in preference to iron in preference to zinc. This follows the inverse order of the metals in the reactivity series. At this level it is worth referring only to the reactivity series of metals as opposed to the electrochemical series, which is linked to electrode potentials.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet