Use this demonstration to find the value of the Faraday constant from electrolysis of copper(II) sulfate solution
In this experiment, students learn how the value of the Faraday constant – the amount of electric charge carried by one mole of electrons – may be determined by using weighed copper electrodes to carry out the electrolysis of aqueous copper(II) sulfate.
This is a relatively straightforward demonstration to set up and carry out, but there is not much to see while it is taking place. This provides an ideal opportunity to ask students to prepare a suitable results sheet and to explain how the masses of the electrodes, the current passed and the time elapsed can be used to calculate a value for the Faraday constant. The experiment will take around 40 minutes.
Students can be told that the calculations are relevant for industrial applications of electrolysis, for example in the extraction of elements such as sodium and chlorine, and in the electroplating industry.
Equipment
Apparatus
- Eye protection
- Access to a fume cupboard
- Beaker, 250 cm3
- Beaker, 100 or 150 cm3
- DC power pack
- Connecting leads, including two fitted with crocodile clips, x3
- Ammeter, preferably digital and a demonstration model
- Rheostat
- Switch
- Stop clock
- Balance, weighing to +/–0.01 g
- Hot-air blower, such as a hair drier
- Emery paper
- Paper towels or tissues
Chemicals
- Copper foil electrodes, x2 (see note 5 below)
- Copper(II) sulfate solution, about 1.0 M (HARMFUL), 200 cm3
- Propanone (HIGHLY FLAMMABLE, IRRITANT), about 200 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) sulfate solution, CuSO4 (aq), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC027c and CLEAPSS Recipe Book RB031.
- Propanone, CH3 COCH3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC085A.
- These should be 2–3 cm wide and long enough to reach from the bottom of a 250 cm3 beaker to the rim, where they can be folded over and gripped by the crocodile clips on the leads.
Procedure
- Clean the copper electrodes with emery paper, rinse under the tap and dry thoroughly using paper towels and a hot-air blower.
- Mark the electrodes as + or – at one end, and weigh them separately. Record the masses.
- Set up the circuit as shown in the diagram, clamping the electrodes to the rim of the beaker using the crocodile clips. Make sure the electrodes do not touch.
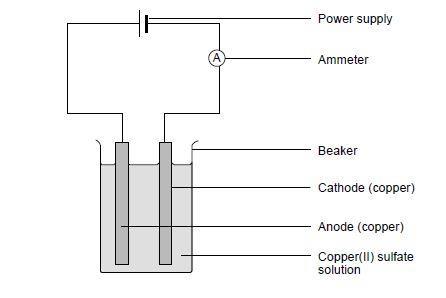
- Fill the beaker with copper(II) sulfate solution to just below the crocodile clips.
- Start the stop clock and switch on the current, setting the rheostat so that a current of 0.50 A passes through the solution.
- The current alters slightly throughout the electrolysis, so it is important to make continual adjustments to the rheostat to keep the current constant.
- After about 30 minutes switch off the current and stop the clock.
- Remove the electrodes from the electrolyte, wash them carefully under running water, then rinse them in a beaker of propanone in a fume cupboard. Finally dry them by allowing the propanone to evaporate in a well-ventilated laboratory, and away from any naked flames.
- Reweigh the dry electrodes. The results should be entered in a table, including the following values:
| Recorded value | |
|---|---|
| Mass of cathode (-) before /g | |
| Mass of cathode (-) after /g | |
| Change in cathode’s mass /g | |
| Mass of anode (+) before /g | |
| Mass of anode (+) after /g | |
| Change in anode’s mass /g | |
| Current used /A | |
| Time for electrolysis /s |
Teaching notes
It is difficult to avoid fluctuations in current throughout the electrolysis, and thus obtain an accurate value to use in the calculations.
Be careful to avoid any movement of the electrodes during the electrolysis.
Thorough washing and drying of the electrodes is vital. A tiny amount of moisture left on the electrodes causes a considerable error in the mass calculations. The electrodes should NOT be heated in a flame to dry them as the copper will oxidise on the surface to form black copper(II) oxide. After initial drying by evaporation of the propanone, a hot-air blower could be used.
Use the student worksheet available for download below to work through the process of calculating the Faraday constant. Students could also be asked to use the official value of 96500 Coulombs per mole to calculate the percentage error.
Answers to student questions
The answers for questions 1–4 are based on students’ experimental observations within the experiment above.
- The calculated value for the Faraday constant should be correct to within about 10%. The major error comes from the value for the current, which is hard to keep constant to two significant figures.
- The reaction at the cathode is: Cu2+(aq) + 2e–→ Cu(s)
- The concentration of Cu2+ ions in solution remains constant. In a given time as many of these ions are produced at the anode as are being removed at the cathode.
- The coating of solid copper on the cathode tends to be ‘spongy’ and some can be lost when electrode is washed or dried. Some may also have fallen off the cathode during electrolysis. Thus the mass gain of the cathode is an unreliable measure of the quantity of copper actually deposited there.
Downloads
Quantitative electrolysis of aqueous copper(II) sulfate worksheet
Editable handout | Word, Size 56.59 kbQuantitative electrolysis of aqueous copper(II) sulfate worksheet
Handout | PDF, Size 0.21 mb
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet