In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt
Using the procedure below, it should take no more than 30 minutes to produce the filtered salt solution.
Experimental work can begin without delay if the dilute sulfuric acid and copper(II) oxide powder are provided in ready-measured quantities (see Health, safety and technical notes).
This procedure can be used by students. A demonstration aided by students may be more sensible if there are real doubts about safe behaviour or adequate manipulative skills.
Equipment
Apparatus
- Eye protection
- Glass beaker, 100 cm3
- Conical flask, 100 cm3
- Spatula
- Glass stirring rod
- Filter funnel (note 1)
- Filter paper (note 2)
- Bunsen burner
- Tripod
- Gauze
- Heat resistant mat
- pH or litmus paper
Apparatus notes
- Polythene filter funnels are safer and cheaper than glass funnels. Filter funnel diameter is important – too large a funnel makes the filtration set-up unstable.
- Filter paper size when folded should match funnel size. Student grade filter paper is adequate.
Chemicals
- Dilute sulfuric acid, 0.5 M (IRRITANT), 20 cm3
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), about 1 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Be very careful not to knock the tripod while the beaker is on it. Consider clamping the beaker.
- When heating the copper(II) oxide and dilute sulfuric acid, avoid boiling off the water and allowing the copper sulfate to appear and then decompose with excessive heating – this is unsafe. The sulfur dioxide gases are toxic and can cause breathing difficulties.
- In the final (optional) stage of the procedure, do not attempt to evaporate the acid to obtain crystals by heating with a Bunsen burner after filtering. This action would fill the lab with toxic fumes.
- Provide the reagents in ready-measured quantities to reduce waste and assist lesson organisation. All containers must be clearly labelled.
- Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS TO THE ENVIRONMENT) – see CLEAPSS Hazcard HC026. The copper(II) oxide powder can be provided in approximately 1 g quantities in labelled specimen tubes or plastic weighing boats.
- Dilute sulfuric acid, H2 SO4 (aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC098a. 20 cm3 of the dilute sulfuric acid should be provided in small labelled bottles.
- Copper(II) sulfate, CuSO4 (s), (HARMFUL, DANGEROUS TO THE ENVIRONMENT) – see CLEAPSS Hazcard HC027c.
Procedure
Stage 1
- Add 20 cm3 of the 0.5 M sulfuric acid to the 100 cm3 beaker. Heat carefully on the tripod with a gentle blue flame until nearly boiling. (Be very careful not to knock the tripod while the beaker is on it. Consider clamping the beaker.)
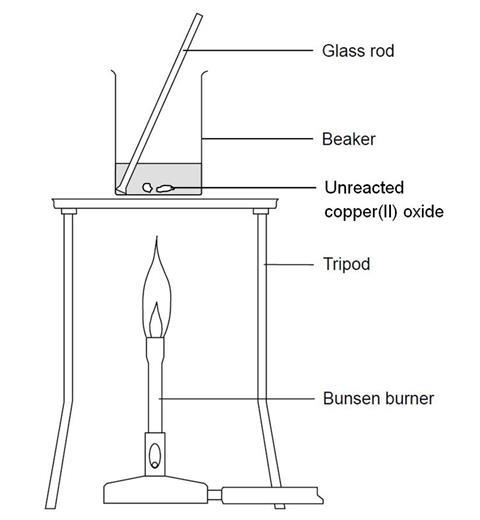
- When the acid is hot enough (just before it starts to boil), use a spatula to add small portions of copper(II) oxide to the beaker. Stir the mixture gently for up to half a minute after each addition. (When adding the solid to the beaker, take care to avoid knocking the beaker.)
- When all the copper(II) oxide has been added, continue to heat gently for 1–2 minutes to ensure reaction is complete. Then turn out the Bunsen burner. It may be wise to check (using pH or litmus paper) that no acid remains. If the acid has not been hot enough, excess acid can co-exist with copper oxide. (Boiling off the water so that the copper sulfate appears and then decomposes with excessive heating is unsafe. The sulfur dioxide gases are toxic and can cause breathing difficulties.)
- Allow the beaker to cool slightly while you set up Stage 2.
Stage 2
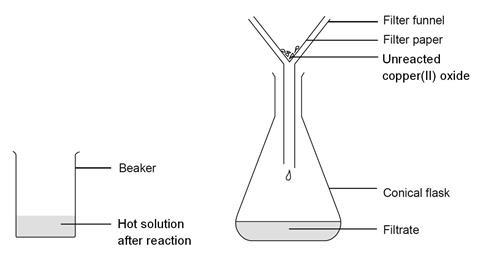
- Place the filter funnel in the neck of the conical flask.
- Fold the filter paper to fit the filter funnel, and put it in the funnel.
- Make sure the beaker is cool enough to hold at the top. The contents should still be hot.
- Gently swirl the contents to mix, and then pour into the filter paper in the funnel. Allow to filter through.
- A clear blue solution should collect in the flask. If the solution is not clear, and black powder remains in it, you will need to repeat the filtration.
Stage 3 (optional)
- Rinse the beaker, and pour the clear blue solution back into it. Label the beaker with your name(s). Leave the beaker in a warm place, where it won’t be disturbed, for a week or so. This will enable most of the water to evaporate. (Do not attempt to evaporate the acid by heating with a Bunsen burner after filtering. The lab would fill with toxic fumes.)
- Before all the water has evaporated, you should find some crystals forming on the bottom of the beaker. Filter the solution. Collect the crystals from the filter paper onto a paper towel.
Teaching notes
Practical points
The safety warnings in stage 1 of the procedure are particularly relevant to younger or more inexperienced students.
Be aware of the problems associated with younger or inexperienced students heating beakers perched on tripods, and with lifting hot glassware off a hot tripod after heating.
For lifting the hot beaker, the provision of beaker tongs of suitable size is a good solution. But many schools will not have these. Do not be tempted to use ordinary tongs. If there is any doubt about the safety of this step, the teacher should lift each beaker down onto the heat-resistant mat.
Chemistry notes
Most metal oxides react with dilute acids. Soluble metal oxides and hydroxides are called alkalis, and react with acids in solution. Most metal oxides are insoluble solids. The reaction between an insoluble metal oxide and a dilute acid is often quite slow so it is possible to observe the progress of the reaction as the solid reactant disappears as a soluble product is formed.
In stage 1, students should be able to observe the colour change from colourless to blue, at the same time as the black powder disappears. The blue colour intensifies as more black powder is used.
In stages 2 and 3, younger students should be able to use their previous experience of blue solutions/crystals to recognise the familiar colour of copper sulfate. This can then be used as the starting point for teaching about acid + metal oxide → salt + water reactions.
Older students, already familiar with acid/base reactions, should be able to predict the identity of the compound formed, using the colour change as confirmation of that prediction.
The symbol equation for the reaction is:
CuO(s) + H2SO4 (aq) → CuSO4 (aq) + H2O(l)
Otherwise, a simple word equation will be sufficient.
Note that there is no easy way of demonstrating that water is the other product.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet