Explore an application of electrolysis in this demonstration by anodising aluminium to improve its corrosion resistance
This experiment illustrates an interesting use for electrolysis. Students observe how anodising aluminium makes the oxide layer on its surface thicker, improving the metal’s resistance to corrosion. In the process, students learn how the thickened oxide surface coating can also be coloured by using dyes.
This works well as a class demonstration, but there are several tasks to complete in preparation. The anodising process itself takes about 30–40 minutes, with nothing particularly dramatic happening, so you will need to plan other activities to fill the time.
At the start of the experiment, show the students the effervescence due to the hydrogen evolved from the cylindrical aluminium cathode. A flexicamera connected to a projector could be used here.
During the anodising phase, the theory could be explained with an emphasis on the applications of the process. A collection of anodised objects such as saucepan lids or sports equipment could be available to look at.
A well-disciplined and organised class might be able to carry out this process for themselves (in twos or threes), but it is strongly recommended that the treatment with sodium hydroxide solution (CORROSIVE) – prior to the electrolysis – is carried out under strict supervision.
Equipment
Apparatus
- Eye protection (goggles)
- Low-voltage DC power pack, adjustable up to 10 V (see note 9 below)
- Connecting leads
- Crocodile clips, x4
- Blu-Tak
- Paper clips, plastic
- Test tube holder, wooden
- Paper tissues
- Strip of wood, 15 cm long
- Ruler, 30 cm
- Beaker, 1 dm3
- Beakers, 250 cm3, x3
Chemicals
- Aluminium foil, approximately 40 cm x 15 cm (see note 3 below)
- Congo Red dye (TOXIC)
- Ethanol (HIGHLY FLAMMABLE, HARMFUL)
- Dilute sulfuric acid, approximately 2M (CORROSIVE), 1 dm3
- Sodium hydroxide, approximately 1.5M (CORROSIVE), 250 cm3
- Propanone (acetone) (HIGHLY FLAMMABLE, IRRITANT) (see note 8 below)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection (goggles) throughout.
- Aluminium foil, Al(s) – see CLEAPSS Hazcard HC001A. Reasonably thick aluminium foil should be used, but, if unavailable, ordinary kitchen foil works quite well.
- Congo Red dye, both the solid and the solution are TOXIC – see CLEAPSS Hazcard HC032. Mix 0.5 g of dye with 50 cm3 of ethanol and 50 cm3 of water and warm to dissolve. As an alternative, Dylon cold fabric dye (Camilla A16) also gives good results. Red fountain pen ink can be used as an alternative but does not give such good results.
- Ethanol, C2 H5 OH(l), (HIGHLY FLAMMABLE, HARMFUL) – see CLEAPSS Hazcard HC040A.
- Dilute sulfuric acid, H2 SO4 (aq), (CORROSIVE) – see CLEAPSS Hazcard HC098a and CLEAPSS Recipe Book RB098.
- Sodium hydroxide solution, NaOH(aq), (CORROSIVE) – see CLEAPSS Hazcard HC091a.
- Propanone (acetone), CH3 COCH3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC085A. Propanone is needed to degrease the aluminium foil and it is worth keeping a bottle specifically for this purpose. The used propanone can be poured back into the bottle and kept for future use. This reduces waste disposal requirements.
- Instead of a power pack, a battery or series of batteries could be used
Procedure
Before the demonstration
- Cut two pieces of aluminium foil, one 10 cm x 3 cm (the anode), the other about 30–35 cm x 12 cm (the cathode). Ensure that when the foil is folded into a cylinder it fits inside the beaker as shown below.
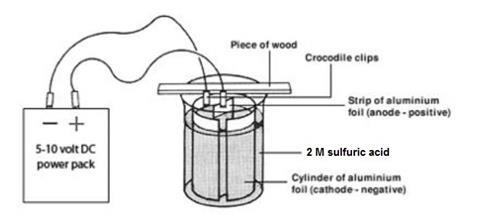
- Work in a fume cupboard and ensure that there are no flames close by. Work on a clean surface. Degrease the two pieces of foil by rubbing well with a paper tissue soaked in propanone (HIGHLY FLAMMABLE, IRRITANT) and then dip the strips into a beaker of propanone for a few seconds.
- Remove the strips of aluminium from the propanone and allow to dry. From this point on, only hold the aluminium foils at the top edges.
- Arrange the larger piece of aluminium into a cylinder. Fix it in position with plastic paper clips and then place inside the large beaker as shown in the diagram.
- Set up the strip of wood on the beaker and use Blu-Tack to attach two crocodile clips, one at the edge and one in the centre. Attach the outer clip to the aluminium cylinder. This is the cathode.
- Pour some of the cold sodium hydroxide solution (CORROSIVE) into a 250 cm3 beaker. Hold the smaller piece of aluminium foil with a wooden test tube holder, and dip it into the sodium hydroxide solution. After a short while, hydrogen gas will be given off rapidly. Remove the strip after a few seconds of fizzing, and wash it in a stream of cold running water.
- Attach the aluminium strip to the central crocodile clip ensuring that it is arranged vertically (see diagram). This central strip (the anode) must not touch the aluminium cylinder.
- Carefully fill the beaker with the sulfuric acid from a measuring cylinder up to a level about 1 cm below the top of the aluminium cylinder.
Health and safety note
Remember that hydrogen (HIGHLY FLAMMABLE) will be evolved during the electrolysis. Keep all naked flames well away from the experiment (eg when heating the dye solution).
Demonstration
- Connect up the circuit and use a voltage of 5–10 volt. Electrolysis is occurring when bubbling can be seen at the cathode (hydrogen). Pass a current for about 20 minutes, or longer, if time permits.
- While the electrolysis is running, heat the dye solution in a beaker to about 70 °C. An electric hotplate is preferable to a Bunsen burner. An additional beaker of boiling water will also be needed.
- Remove the central aluminium strip (the anode) and place it in the hot dye solution. Stir and leave for about 10–15 minutes.
- Transfer the aluminium anode to a beaker of boiling water and leave for another 10 minutes. This seals the dye onto the anodised surface of the aluminium and makes the aluminium oxide layer less porous.
- The upper non-anodised portion of the strip should be the original metallic grey colour whilst the rest should be coloured red. The aluminium strip can be dried in paper tissue and passed round the class. It should not be possible to rub off the dye off the surface.
Teaching notes
Carrying out the demonstration
The instructions may seem very detailed, but experience shows that success depends on getting the conditions just right. You should try out the experiment before carrying it out as a demonstration. It would be useful to have some sample strips of anodised aluminium to pass round.
The voltage will drop during the experiment, since the anode is becoming increasingly coated with aluminium oxide. If a rheostat and voltmeter are used, the readings can be constantly monitored and adjustments made to keep the voltage approximately constant.
A longer immersion in the dye will produce a strip with a deeper red colour. Leaving the strip in the dye overnight produces the best results.
If time is short, omit the dye-sealing stage in boiling water.
If there is time, a piece of the cathode could also be immersed in the dye. It will be found that the dye is not taken up by the metal in the same way.
This is a good experiment to show students towards the end of their study of electrolysis.
Background information and theory
When a piece of aluminium is exposed to the air, it rapidly becomes coated with a protective surface layer of aluminium oxide.
Heating the aluminium in air can make the oxide layer thicker, but anodising is much more effective.
The oxide layer can be made to absorb dyes. This is useful in a range of everyday goods, such as kettles, window frames and some sports equipment, all of which need to be able to withstand extreme physical conditions.
Untreated aluminium has an oxide layer about 10–8 m thick. This explains aluminium’s apparent lack of reactivity in the laboratory. Anodising thickens this layer to about 10–5 m and dramatically improves the metal’s corrosion resistance.
Theory for able students
Oxygen is often evolved at the anode during the electrolysis of aqueous solutions. Aluminium is a reactive metal. The oxygen formed reacts immediately with the aluminium. It forms a solid oxide coating on the surface of the metal electrode.
Theory for more able students
For students working at a higher ability level, some or all of the following equations and explanations could be introduced:
The cleaning process with NaOH:
- Al2O3(s) + 2NaOH(aq) + 3H2O(l) → 2NaAl(OH)4(aq)
- 2Al(s) + 2NaOH(aq) + 6H2O(l) → 2NaAl(OH)4(aq) + 3H2(g)
Electron transfer occurring at the anode (+):
6OH‾(aq) + 2Al(s) → 3H2O(l) + Al2O3 (s) + 6e‾
Electron transfer occurring at the cathode (–):
6H+(aq) + 6e– → 3H2 (g)
Negative hydroxide ions, OH–, are destroyed (discharged as oxygen) at the surface of the anode. Therefore H+ ions predominate in the solution immediately around the anode. The solution around the aluminium strip is acidic (due to H+ ions) and the oxide coating therefore develops a positive charge. The oxide coating attracts dyes which contain a coloured anion. The negatively-charged dye molecules are absorbed in the pores of the sponge-like oxide layer.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
Health and safety checked, 2016


















No comments yet