Try these activities to identify your students’ mathematical misconceptions when presenting chemistry data. Use them in your teaching to help students interpret their practical observations using non-linear graphs.
Many of the graphs pre-16 chemistry students experience will be non-linear, as they show the progression of a reaction. Students must be able to relate the graph to what is happening during the experiment and then try to offer an explanation.
Example 1 - Changes of state
Working through this simple exercise and then encouraging the students to use their answer to ask each other questions will help them to consolidate their ideas and enable them to progress to writing full explanations.
Look at the graph below where ice is being heated from -10 °C to 110°C.
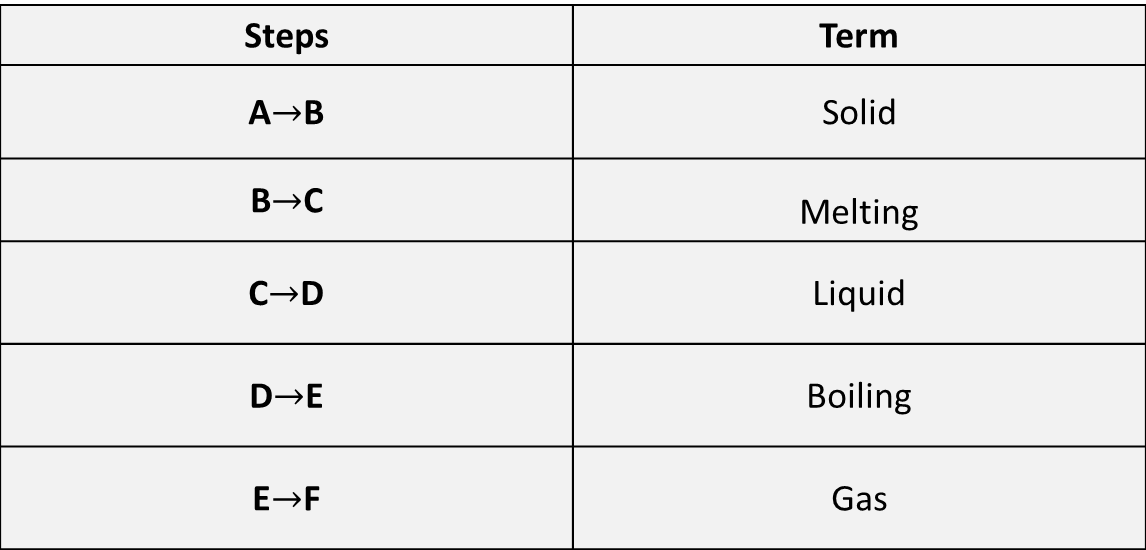
From the options below, choose a term that best describes the steps being shown from A→E.
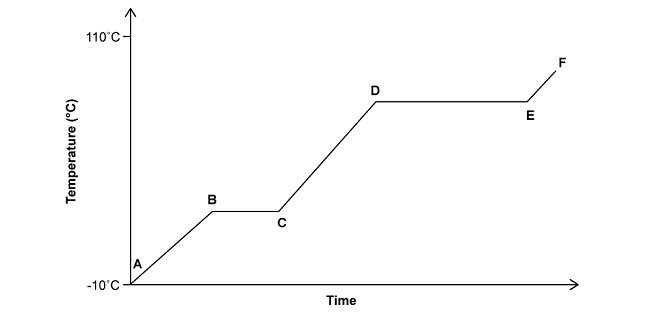

Once you have identified your answers, head to the bottom of this page for confirmation.
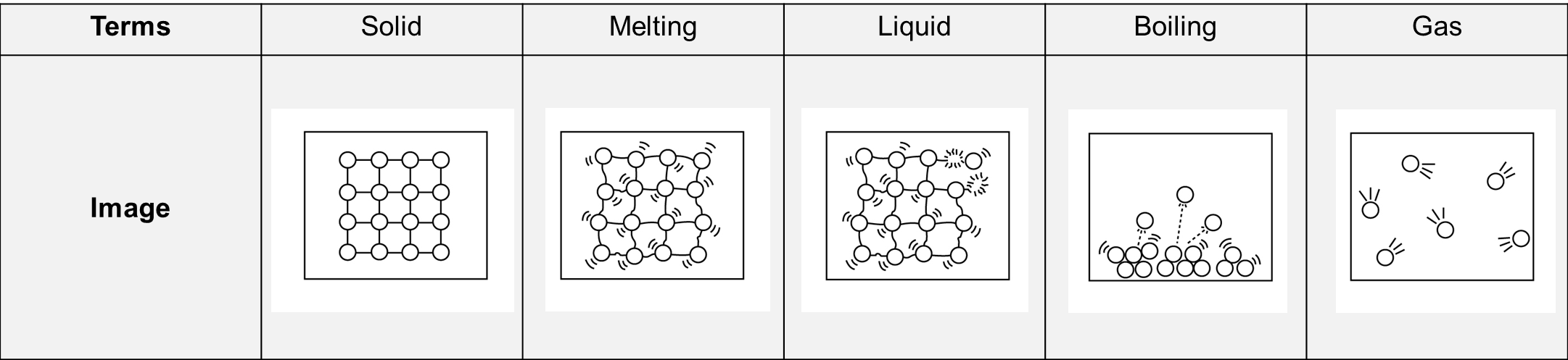
Now look at the graph again where ice is being heated from -10 °C to 110°C.
Choose an image that best describes the phase or process that is happening at each stage in the graph.


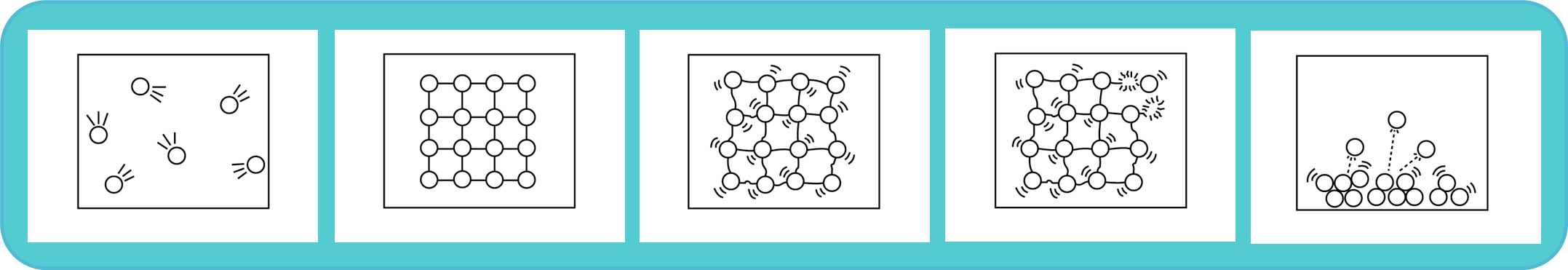
Once you have identified your answers, head to the bottom of this page for confirmation.
Example 2 - Rates of reaction
Understanding how the gradient of a non-linear graph changes will help students make links to what is happening in the reaction. Notice how the gradient of the graph changes during a typical chemical reaction such as that between marble chips and hydrochloric acid.
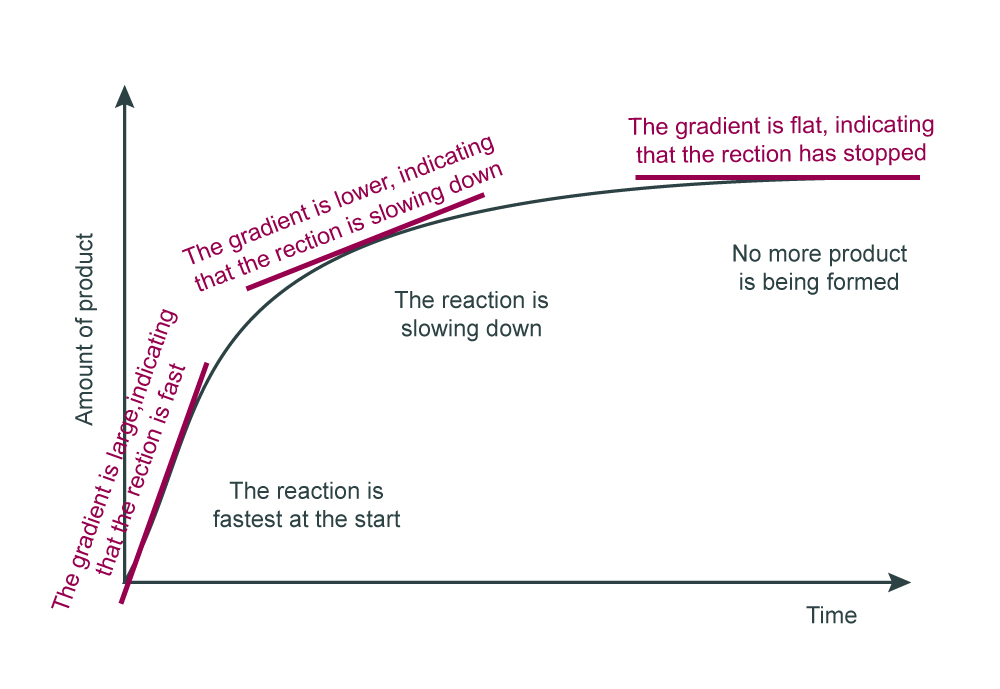
If students can appreciate what the gradient means, it will help them to work out what is happening in the reaction vessel.
Example 3 - Matching graphs
The matching graphs and statements activity is a useful activity to do with your students. Here the students must focus on the actual graphs as they use them to explain what story they tell.
Answers
Additional information
These examples appear in our free Maths Skills online CPD course which offers 188 different exercises like this to help with applying mathematical ideas to chemical contexts.
The course has nine topics including how to use Algebra, Units and Decimals and significant figures and will take nine hours to complete.
You can find further details about the structure of the course in the additional information below:
After working through our Maths Skills course you will be able to:
- become more confident in your own understanding of the maths used by chemists;
- appreciate how the mathematical ideas develop and progress throughout secondary education;
- develop an understanding of the difficulties faced by students when trying apply mathematical ideas to chemical problems;
- become more confident and competent in teaching mathematical aspects of chemistry to secondary aged students; and
- be equipped with a range activities and resources to support students in using maths in chemistry.
Visit our teacher CPD pages to view our other courses.
Thank you to Dorothy Warren and Derek Denby for authoring this course.


















No comments yet