Double-twist Möbius Aromaticity in a 4n+2 Electron
Electrocyclic Reaction
Henry S. Rzepa
Department of Chemistry, Imperial College London, South
Kensington Campus, Exhibition Road, London, SW7 2AY
Electronic Supporting Information
The models below use the Jmol
Java-based applet for visualisation of molecular geometries
and animations. To ensure this runs correctly, please install
Java JRE Version 1.4.2_5 or higher on your
computer, and ensure that the local security policy enables
applet display. The coordinates for each computed stationary
point are in CML (Chemical Markup
Language), MDL Molfile or XYZ animation form.
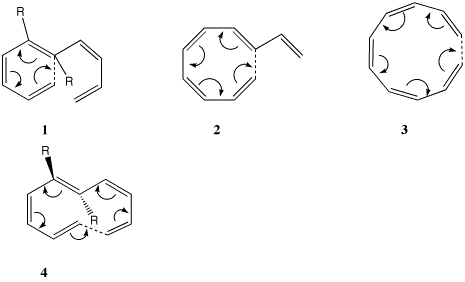
| Table S1. Calculated (B3LYP/6-31G(d) )
Geometries and transition normal modes for
the electrocyclic ring openings 1-4. |
| 1, R=H
| 2, R=H
|
| |
|
| 3, R=H
|
4, R=H
|
| |
|
| Double-twist Mobius cyclacene band7 | Triple-twist Mobius cyclacene band7 |
| |
|
| 1, R=tBu
| 4, R=tBu
|
| |
|
4, R=tBu, Reactant
| 4, R=tBu, Product
|
| |
|
| 4, R=(R,R)-CHFCl
| 4, R=(S,S)-CHFCl
|
| |
|
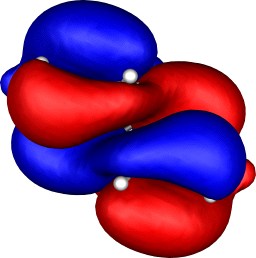
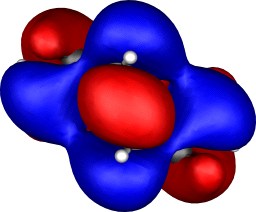
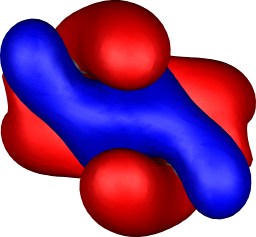
Molecular orbitals
To demonstrate the 3D nature of the orbitals, each thumbnail
image is linked to a 3DMF file. To view these orbital models,
you will need a 3DMF viewer such as
3DMFPlugin (a browser plugin), 3DMF
Optimizer or Geo3D (Macintosh applications)
or 3DMF
Viewer for Windows. To install a Browser plugin for use
under Windows;
- install the
QuickDraw3D libraries from Apple
- Download this plugin
- Unzip the contents of NPQuick3D32.ZIP and copy the single
file (npquick3.dll) to the plugins directory of your browser.
The path to this will be something like C:\Program
files\Mozilla\Plugins. We recommend a browser such as Mozilla
or FireFox. Internet Explorer no longer supports such
plugins, and should not be used for this purpose.
| Table S2. Calculated five highest energy molecular orbitals for 3
at the B3LYP/6-31G(d) level, contoured at 0.01 au. |
 |
 |
 |
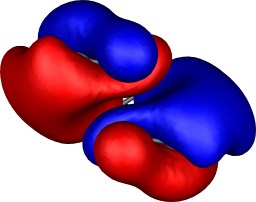 |
 |
References
- E. Heilbronner, Tetrahedron Lett., 1964,
1923-8.
- H. E. Zimmerman, J. Am. Chem. Soc., 1966,
88, 1564-5.; H. E. Zimmerman, Accounts Chem
Res., 1971, 4, 272-80.
- H. Jiao, P. v. R. Schleyer, Angew. Chem., Int.
Ed., 1993, 32, 1763-5; H. Jiao, and P. von
R. Schleyer, J. Chem. Soc, Perkin Trans. 2,
1994, 407-10.
- H. S. Rzepa, Chem. Rev., 2005, in press.
- D. Ajami, O. Oeckler, A. Simon and R. Herges,
Nature, 2003, 426, 819-21.
- H. M. Sulzbach, H. F. Schaefer, K. Klopper, and H. P.
Lüthi, J. Am. Chem. Soc., 1996,
118, 3519-20; R. A. King, T.D. Crawford, J. F.
Stanton, and H. F. Schaefer, J. Amer. Chem. Soc.,
1999, 121, 10788-93; H. S. Rzepa, N. Sanderson,
Phys. Chem. Chem. Phys, 2004, 6,
310-3.
- M. Martin-Santamaria and H. S. Rzepa, J. Chem. Soc.;
Perkin Transactions 2, 2000, 2378-81.
- P. von R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao,
and N. J. van Eikema Hommes, J. Amer. Chem. Soc.,
1996, 118, 6317-8; P. v. R. Schleyer, M.
Manoharan, Z. Wang, X. B. Kiran, H. Jiao, R. Puchta, and N.
J. van Eikema Hommes, Org. Lett., 2001, 3,
2465-8; C. Corminboeuf, T. Heine, T. Gotthard, P. von R.
Schleyer, J. Weber, Phys. Chem. Chem. Phys.,
2004, 6, 273-6.
- J. K. Kang and C. B. Musgrave, J. Chem. Phys.,
2001, 115, 11040-51; H. J. P. Senosiain, C. B.
Musgrave and D. M. Golden, Faraday Discussions,
2001, 119, 173-89.
- C. S. Wannere and P. v. R. Schleyer, Org. Lett.,
2003, 5, 865-8; C. S. Wannere, K. W.
Sattelmeyer, H. F. Schaefer, P. von R.Schleyer, Angew.
Chemie, Int. Ed., 2004, 43, 4200-6.
- H. S. Rzepa, Org. Lett., 2005, 7, in
press.










