Additions and corrections
The synthesis of amines by the homogeneous hydrogenation of secondary and primary amides
Angel A. Núñez Magro, Graham R. Eastham and David J. Cole-Hamilton
Chem. Commun., 2007, 3154-3156 (DOI: 10.1039/b706635j) Amendment published 14th November 2011
Authors of this correction: Deborah L. Dodds, Jacorien Coetzee, Jürgen Klankermayer, Sandra Brosinski, Walter Leitner and David J. Cole-Hamilton
Although the work published in this paper proved reproducible by at least three different researchers in the St. Andrews laboratories, it could not be reproduced elsewhere, nor could it be reproduced in St. Andrews when we changed to a different batch of 1,1,1-tris(diphenylphosphinomethyl)ethane (triphos).
Subsequent study has now produced a protocol which is reproducible not only in our hands but also in the laboratories of Professor Walter Leitner and Dr. Jürgen Klankermeyer at RWTH, Aachen. Full details of the new procedure will be published elsewhere, but we report below an example for the hydrogenation of acetanilide as carried out in St. Andrews and Aachen.
Autoclave
The reaction is best carried out in a HastelloyTM autoclave, but the work in Aachen was carried out in glass lined stainless steel autoclaves. Larger autoclave volumes are preferred if reactions are carried out in a closed system to avoid problems with gas depletion, but these can be overcome by working at higher pressure.
Procedure 1 (St. Andrews)
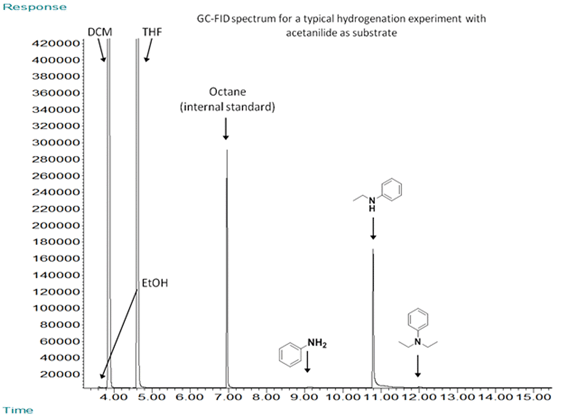
Acetanilide (0.68 g, 5 mmol), [Ru(acac)3] (0.02 g, 0.05 mmol, 1 mol%) and triphos (0.062 g, 0.10 mmol, 2 mol%) were weighed into a 250 ml HastelloyTM autoclave in air. A new Teflon O-ring was fitted, a stirrer bar added and the autoclave sealed, attached to the Schlenk line and degassed by three vacuum / N2 cycles. A septum was fitted and a solution of methanesulfonic acid (4.87 μl, 0.075 mmol, 1.5 mol%) in dry degassed THF (10 ml) was added. The autoclave was purged with hydrogen (~10 bar, 6 times) and pressurised to 10 bar with hydrogen. The autoclave was sealed and leak checked, then heated to an internal temperature of 200°C with a heating jacket and magnetic stirring. Once the reaction was complete (16 h), the autoclave was cooled rapidly by immersing in cold water. The cooled autoclave was vented carefully to the atmosphere in a well-ventilated fumehood. The contents were then transferred to a sample vial and the crude mixture analysed by GC-MS, GC-FID and NMR spectroscopy. The major product was N-ethylaniline (97%) with traces of ethanol, and N,N-diethylaniline.
Procedure 2 (Aachen)
Reaction procedure and conditions: 10 ml stainless steel autoclave with glass insert.
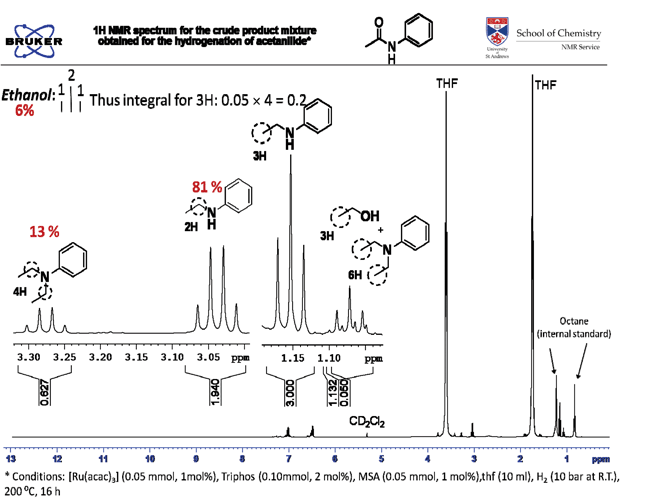
Acetanilide (338 mg, 2.5 mmol), [Ru(acac)3] (10.0 mg, 0.025 mmol, 1 mol%) and triphos (31.0 mg, 0.05 mmol, 2 mol%) were transferred to a glass insert in air. A stirrer bar was added and the glass insert placed in a 10 ml-autoclave. The autoclave was degassed (3 times). Subsequently, degassed THF (4.0 ml) and methanesulfonic acid (3.7 mg, 0.038 mmol, 1.5 mol%) as a solution in THF (1.0 ml, stock solution 3.7 mg per ml) were added via a syringe under a flow of argon. The autoclave was purged with hydrogen (3 times) and pressurised to 50 bar. Then the system was heated to an internal temperature of 200°C (heating jacket) and stirred for 16 h. After the reaction, the autoclave was cooled to room temperature and carefully vented to atmosphere. The resulting orange solution was analysed by NMR. The conversion was 96.6 % with selectivity to N-ethylaniline of 87.7%. Small amounts of ethanol and N,N-diethylaniline were also observed.

Below we show an NMR spectrum of a product from a slightly less selective reaction so that we can show the resonances that arise from the product and side products, ethanol and N,N'-diethylaniline.
The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.
Back to article