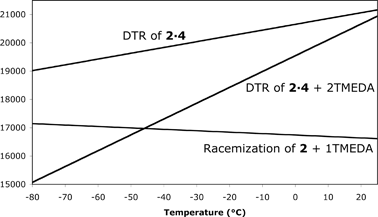
Fig. 1 The relationship between ΔG‡ and temperature for the three systems studied (Table 1). For the DTRs, the barriers are for R-2 → S-2.
Additions and corrections
The barrier to enantiomerization and dynamic resolution of N-Boc-2-lithiopiperidine and the effect of TMEDA
Iain Coldham, Daniele Leonori, Timothy K. Beng and Robert E. Gawley
Chem. Commun., 2009, 5239-5241 (DOI: 10.1039/b911024k) Amendment published 10 March 2010
In the original manuscript and supplementary information, the kinetics equations inadvertently included a factor of 0.5. This error affected the subsequent calculations of rate constants. The supplementary information has been corrected and replaced. In addition, a nonlinear fit to zero order plots has now been used to calculate all the rate constants, which, in turn, afforded the revised inversion parameters listed below.
The corrected data for the manuscript are as follows:
| Entry | Description | ΔH‡/kcal mol−1 | ΔS‡/cal mol−1 K−1 |
|---|---|---|---|
| 1 | Enantiomerization of 2 with 1 equiv. TMEDA | 18.1 ± 0.7 | 5.0 ± 3.2 |
| 2 | DTR of 2 with 1 equiv. 4 | 15.1 ± 0.4 | −20.4 ± 1.4 |
| 3 | DTR of 2 with 4 and 2 equiv. TMEDA | 4.3 ± 0.5 | −55.9 ± 1.8 |

Fig. 1 The relationship between ΔG‡ and temperature for the three systems studied (Table 1). For the DTRs, the barriers are for R-2 → S-2.
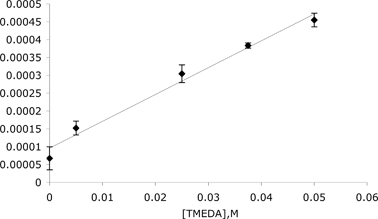
Fig. 2 Observed rate constants for DTR of 2 by 4 at −10 °C vs. [TMEDA].
The text supporting Figure 2 should read:
The intercept of 10.0(±0.2) × 10−5 s−1 indicates a competing DTR having a zero-order dependence on TMEDA (i.e., DTR not catalyzed by TMEDA), and is similar to the experimental value of 6.75(±0.3) × 10−5 s−1 °C (see ESIć). The rate constant of the DTR in Et2O at −10 °C in the presence of 1.0 equiv. TMEDA, k = 4.55(±0.33) × 10−4 s−1, is over six times that in the absence of TMEDA.
The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.
Back to article