We have previously reported the formation of diglycosylated isoindolin-2-ones by Larock-iodocyclization of the corresponding o-alkynylbenzamides. Further investigations revealed however that isobenzofuran-1(3H)-imines are formed instead. This is not caused by the steric bulk of the glycosyl residues but rather represents the general reactivity of the substrate class. Thus, a revision of our previously reported structures is necessary.
Background
The iodocyclization products 24 and 25 reported earlier by us were liquids and their structural assignment was based on 2D NMR data and comparison of the chemical shifts with known compounds (T. Yao, R. C. Larock, J. Org. Chem. 2005, 70, 1432–1437). After publication of our results, a crystalline product was obtained by introduction of a nitro group into the aromatic system. X-Ray crystallography revealed that the originally assumed cyclization onto amide nitrogen did not occur and that the amide oxygen was attacked instead. The NMR data of this product were highly similar to those of 24 and 25 and since standard 1H 13C correlations had obviously led to a wrong assignment, we recorded 1H 15N HMBC data to find all three products being inconsistent with a lactam structure. Further examination of simpler iodocyclization products reported in the literature by NMR and X-ray crystallography revealed that imidate formation is the rule rather than the exception. A discussion of the course of the reaction can be found in J. Org. Chem., 2012, 10118–10124, a correction of Scheme 5 in our original publication is given below.
Correction
In Scheme 5, the structures of compounds 24 and 25 have been corrected.
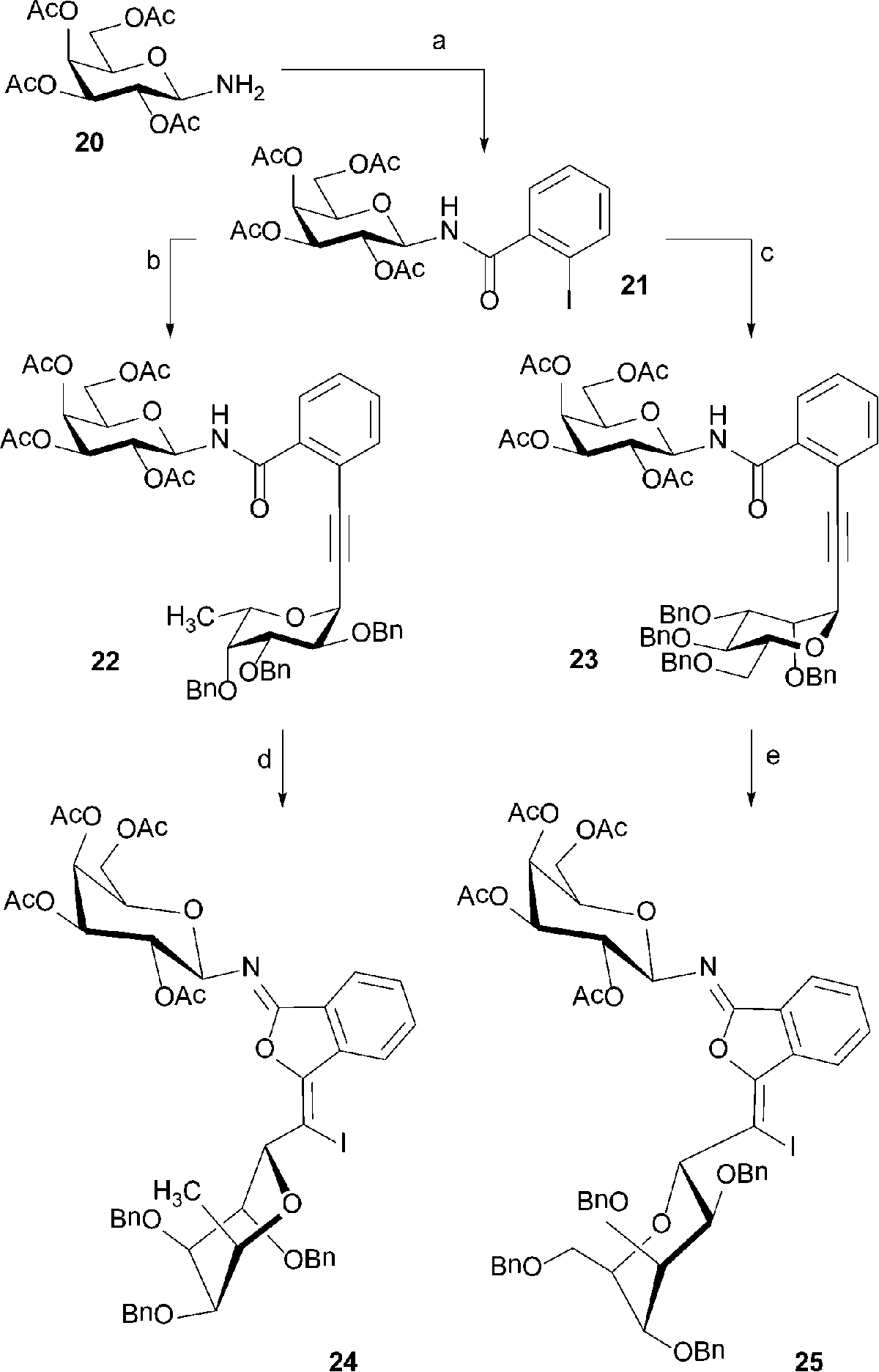
Scheme 5 Reagents and conditions: (a) 2-iodobenzoyl chloride, N-ethylmorpholine, THF, 0 °C, 90%; (b) 17, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 80 °C, 60%; (c) 12, Pd(PPh3)2Cl2, CuI, Et3N, DMF, 60 °C, 76%; (d) I2, NaHCO3, CH3CN, 25 °C, 76%; (e) I2, NaHCO3, CH3CN, 25 °C 94%.