Additions and corrections
| Phys. Chem. Chem. Phys., 2003, 5 | |
Additions and corrections A disjoining pressure study of n-dodecyl- |
Cosima Stubenrauch, Judith Schlarmann and Reinhard Strey
Phys. Chem. Chem. Phys., 2002, 4, 4504 (DOI: 10.1039/b205728j). Amendment published 25th April 2003
In a recent paper we reported a surface tension isotherm for aqueous
solutions of the nonionic sugar surfactant n-dodecyl-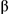 -D-maltoside (
-D-maltoside (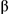 -C12G2).1 Unfortunately, we have made a (small, but
significant) mistake in calculating the concentrations. Instead of a molecular
weight of M = 510.62 g mol–1
for
-C12G2).1 Unfortunately, we have made a (small, but
significant) mistake in calculating the concentrations. Instead of a molecular
weight of M = 510.62 g mol–1
for 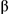 -C12G2,
inadvertently the molecular weight of the corresponding glucoside, namely
n-dodecyl-
-C12G2,
inadvertently the molecular weight of the corresponding glucoside, namely
n-dodecyl-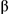 -D-glucoside (
-D-glucoside (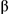 -C12G1), has
been used in ref. 1. As M(
-C12G1), has
been used in ref. 1. As M(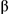 -C12G1) = 348.48
g mol–1 = 0.683 M(
-C12G1) = 348.48
g mol–1 = 0.683 M(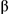 -C12G2) the
concentrations c given in ref. 1 have to be
multiplied by a factor of 0.683. In Fig. 1 presented here
the
-C12G2) the
concentrations c given in ref. 1 have to be
multiplied by a factor of 0.683. In Fig. 1 presented here
the 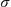 (c)-data, as originally
published,1 are given as full
circles. Multiplying the concentrations by 0.683 leads, due to the logarithmic
scale, to a parallel shift towards lower concentrations at the same respective
surface tensions (hollow circles). In order to be on the safe side we repeated
the measurement of the whole surface tension isotherm once more. The new
experimental results (full triangles) and the previous, now correctly plotted
results of ref. 1 confirm each other within the
experimental error. The slightly lower value of
(c)-data, as originally
published,1 are given as full
circles. Multiplying the concentrations by 0.683 leads, due to the logarithmic
scale, to a parallel shift towards lower concentrations at the same respective
surface tensions (hollow circles). In order to be on the safe side we repeated
the measurement of the whole surface tension isotherm once more. The new
experimental results (full triangles) and the previous, now correctly plotted
results of ref. 1 confirm each other within the
experimental error. The slightly lower value of
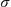 at concentrations above the c.m.c.
is probably due to the use of a different batch of surfactant. However, the
critical micelle concentration cc.m.c. is not
influenced demonstrating the reliability and accuracy of surface tension
measurement in this concentration and surface tension range.
at concentrations above the c.m.c.
is probably due to the use of a different batch of surfactant. However, the
critical micelle concentration cc.m.c. is not
influenced demonstrating the reliability and accuracy of surface tension
measurement in this concentration and surface tension range.

|
||
Fig. 1 Surface tension
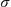 as a function of the surfactant
concentration c for aqueous solutions of n-dodecyl- as a function of the surfactant
concentration c for aqueous solutions of n-dodecyl-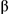 -D-maltoside ( -D-maltoside (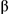 -C12G2) as
published in ref. 1 (full circles), recalculated according
to c = 0.683 cpublished in ref. 1 (hollow circles), and remeasured (full
triangles). Accordingly the absolute error is about a symbol width. The lines
represent fits of the Langmuir–Szyskowski isotherm (eqn.
(1)). -C12G2) as
published in ref. 1 (full circles), recalculated according
to c = 0.683 cpublished in ref. 1 (hollow circles), and remeasured (full
triangles). Accordingly the absolute error is about a symbol width. The lines
represent fits of the Langmuir–Szyskowski isotherm (eqn.
(1)). |
||
As can be seen in Fig. 1, the new curve is in
accordance with the recalculated one. On the basis of these two 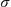 (c)-curves we determine the
c.m.c. to be cc.m.c. = (0.16
± 0.01) mM instead of the erroneous (0.22
± 0.02) mM in ref. 1. The
full lines in Fig. 1 are a description of the surface
tension by a Langmuir–Szyskowski isotherm.
(c)-curves we determine the
c.m.c. to be cc.m.c. = (0.16
± 0.01) mM instead of the erroneous (0.22
± 0.02) mM in ref. 1. The
full lines in Fig. 1 are a description of the surface
tension by a Langmuir–Szyskowski isotherm.
| (1) |
As can be seen from the functional form of eqn. (1),
only the parameter a extracted from the Langmuir–Szyskowski fit is
directly affected by our mistake. The concentration a denotes the point
at which 50% of 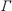 , the
maximum surface concentration, has been reached (see Table
1).
, the
maximum surface concentration, has been reached (see Table
1).
Table 1 Critical micelle
concentration cc.m.c., maximum surface
concentration 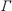 ¥, and concentration a at which
50% of ¥, and concentration a at which
50% of 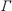 ¥ has been reached; these parameters
were used for calculating the full lines in Fig. 1 ¥ has been reached; these parameters
were used for calculating the full lines in Fig. 1
|
|||
| Surface tension isotherm |
cc.m.c. / mM |
a / 10–6
mol l–1 |
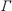 ¥/ 10–6
mol m–2 ¥/ 10–6
mol m–2 |
| Ref. 1 | 0.22 | 8.19 | 4.68 |
| Ref. 1, corrected | 0.15 | 6.09 | 4.82 |
| New measurement | 0.17 | 4.59 | 4.45 |
| |
|||
While we are sorry for the mistake, it is important to underline that
the effect of our mistake is limited to the surface tension isotherm and the
parameter a extracted from the 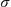 (c)-curve. Although all the
concentrations given in ref. 1 have to be multiplied by
0.683, the shift towards slightly lower concentrations influences neither the
theoretical analysis of the main experimental result of ref.
1, the disjoining pressure
(c)-curve. Although all the
concentrations given in ref. 1 have to be multiplied by
0.683, the shift towards slightly lower concentrations influences neither the
theoretical analysis of the main experimental result of ref.
1, the disjoining pressure  (h)-curves, nor the overall
discussion of both the
(h)-curves, nor the overall
discussion of both the 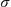 (c)-curve and the
(c)-curve and the  (h)-curves.
(h)-curves.
1. C. Stubenrauch, J. Schlarmann and R. Strey, Phys. Chem. Chem. Phys., 2002, 4, 4504.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.