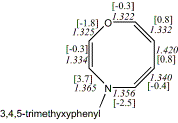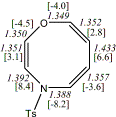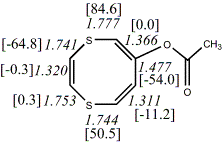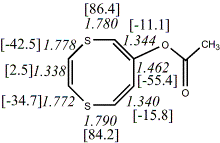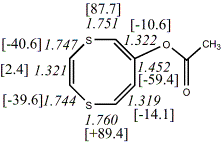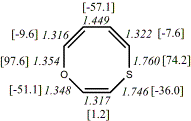B3LYP/KMLYP 6-31G(d) Energy, Hartree
NICS(0), ppm [B3LYP/KMLYP] |
Crystal Geometry:
length, Å
[dihedral, degrees] |
Geometry (B3LYP) |
Geometry (KMLYP) |
4, X=Y=O, R=H
|
-382.59901/
-381.82874 [-8.2/-7.3] |
planar [0.0/00.0] |
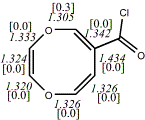
4, X=Y=O17
-724.75782/-723.29019a,b [-7.5/0.1] |
 |
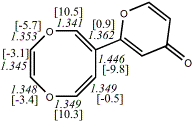
|
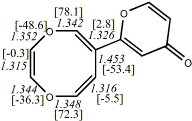 |
4, X=Y=O17
955.53210/-954.11459 [-7.7/-7.0] |
 |
 |
 |
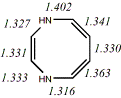
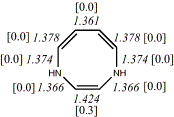
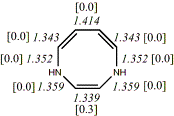
4, X=Y=NH18
-342.89994/-342.21433 [-12.9/-12.5]
|
 |
|
 |
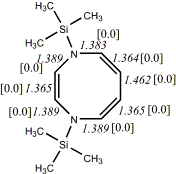
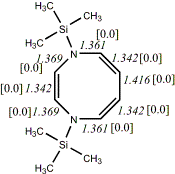
4, X=Y=N-TMS18
-1160.27700/-1158.59120 [-11.3/-10.8]
|
Reported as planar
|
 |
 |
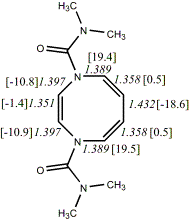
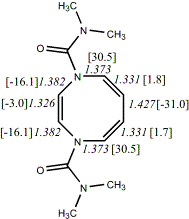
4,
X=Y=N-CONMe219
-837.52547/-835.89800 [-8.5/-6.0]
|
Reported as planar
|
 |
 |
4, X=Y=NCO2Me19
-798.64839/-797.07613 [-6.0/-1.3]
|
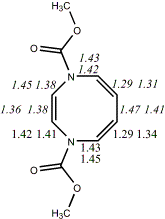 |
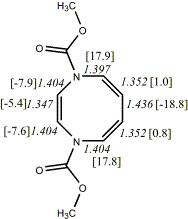 |
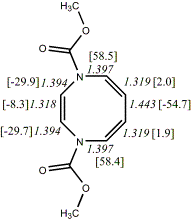 |
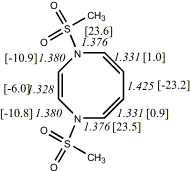
4,
X=Y=N-SO2Me18
-1518.65134/-1516.49681c,d [-9.6/-7.4]
|
Reported as twisted
|
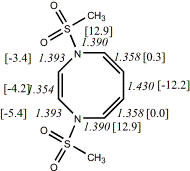
|
 |
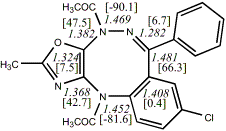
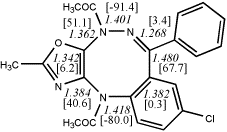
5,
X=Y=N-Acetyl24
-1715.32163/-1712.39694 [-0.8/-0.6]
|
 |
 |
 |
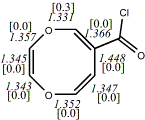
4, X=O, Y=N,
R'=(3,4,5-trimethoxybenzyl)20
-976.66132/-974.75355 [-10.0/-9.2]
|

|
 |
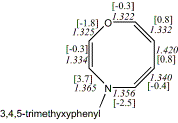 |
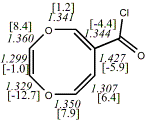
4, X=O, Y=N-Tosyl20
-1181.67739/-1179.74661e,f [-8.9/-7.6]
|
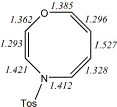
|
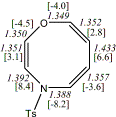 |
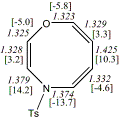 |
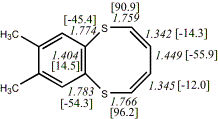
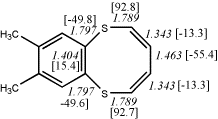
4, X=Y=S21
-1256.41893/-1254.75146 [-2.5/-2.4]
|
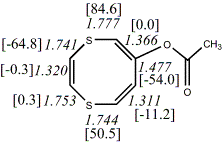 |
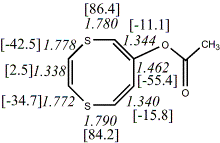 |
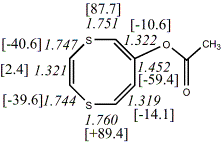 |
4, X=Y=S22
-1260.83295/-1259.16593 [-2.2/-2.0]
|
 |
 |
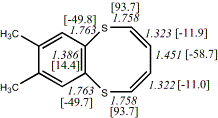 |
4, X=S, Y=O23
-2852.64265/-2849.43451 [-2.17/-2.1]
|
 |
 |
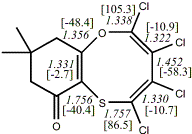 |
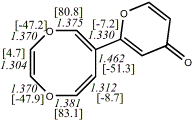
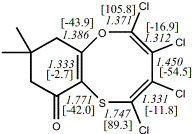
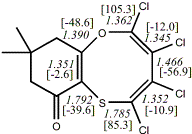
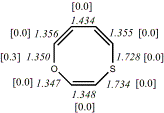
4, X=O, X=S
-705.57218/-704.57509g [-9.2/-1.2 ] |
unknown
|
 |
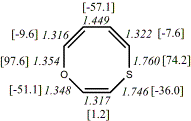 |
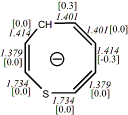
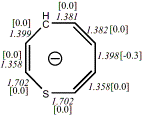
4, X=CH-,
X=S
-669.09512/-668.17759[-14.1/-14.6]
|
Diatropic by NMR25
|
 |
 |
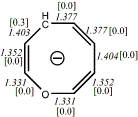
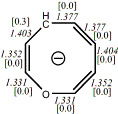
4, X=CH-, X=O
-346.1104603/-345.4187586[-13.5/-13.5]
|
Diatropic by NMR18 |
 |
 |
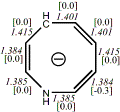
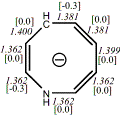
4, X=CH-, X=NH
-326.249677/-325.60088 [-15.8/-16.3]
|
Diatropic by NMR18 |
 |
 |
| 6, X=Y=Z=NH -320.79828/-320.16244 [-8.2/-7.4] |
Unknown |
36.7 |
12.5 |
| aCorrected for DG: -723.15206, Total energy (MP2): -722.61300
bPlanar valence isomer; total energy
(KMLYP/MP2) -723.29133/722.61166. Corrected for DG(KMLYP): -723.14989, NICS(KMLYP) =
-6.1. c Total energy (MP2): -1515.42207 d Non-planar
isomer (MP2); -1515.42046, C3-X4-C5-C6 dihedral =
82.5o. e Corrected for DG: -1179.53934. Total energy (MP2); -1139.55775. fNon-planar
isomer; total energy (KMLYP/MP2) -1179.74319/-1139.55812 Corrected for DG(KMLYP): -1179.53740. NICS(KMLYP)
=-1.2. C3-X4-C5-C6 dihedral (KMLYP) = 82.5o.
gValence isomers; total energy (B3LYP/KMLYP)
-705.56747/-704.57398 [-1.6/-8.6]. Transition state
for interconversion (KMLYP): -704.57242 [-4.1] |