Additions and corrections
Peroxy radical isomerization in the oxidation of isoprene
John D. Crounse, Fabien Paulot, Henrik G. Kjaergaard and Paul O. Wennberg
Phys. Chem. Chem. Phys., 2011, 13, 13607–13613 (DOI: 10.1039/C1CP21330J). Amendment published 6th September 2012.
Equation 4 should read:
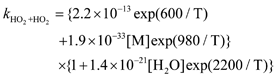
The typographical error in the manuscript display of Equation 4 did not influence the analysis (as can be determined from inspection of Equation 5 and the data presented in Table 1). We thank our colleagues Domenico Taraborrelli, Mark Lawrence, John Crowley, Terry Dillon, Sergey Gromov, Christoph Groß, Luc Vereecken and Jos Lelieveld1 for bringing this typographical error to our attention. The Taraborrelli et al.1 study also revealed a misunderstanding of our analysis. Taraborrelli et al. interpret our study as implying that the Z-1-OH-4-OO- and Z-4-OH-1-OO- 1,6-H-shift isomerization occurs at a rate ~50 times slower than calculated by theoretical methods and then question this result by comparison to extrapolated rates2 for a similar H-shift in 1-hydroxy-4-pentylperoxy. However, we did not measure the rate of the isomerization of the Z-1-OH-4-OO (or Z-4-OH-1-OO) hydroxyperoxy radicals. Our experimental results only speak to the rate of HPALD (HPALD1 + HPALD2) production. The distinction is important for two reasons.
1. Comparisons of our results to those estimated theoretically must, as clearly articulated by Peeters,3 consider the entire pool of peroxy radicals and not simply consider the rate of isomerization of the Z-conformers (as done by Taraborrelli et al.1 ). Just as different levels of theory produce different thermodynamic barriers for the Z-conformer isomerization pathways,1 these different levels of theory will undoubtedly alter the estimate of the fraction of all peroxy radicals in the relevant Z-conformers, and thereby modify the calculated overall rate of the H-shift chemistry.
2. Consistent with Peeters and Müller,4 Peeters et al.,3 and adopted by Taraborrelli et al.,1 we assumed (see last sentence in Section 2.1) that the yield of HPALD from the H-shift chemistry is unity. Recent calculations by Peeters and Nguyen,5 however, suggest an alternative pathway that does not lead to HPALD (see Fig. 1). Analysis of other data from our experiments provides tantalizing evidence for this chemistry. In particular, coincidently with formation of HPALD, we observe signals that are consistent with formation of hydroperoxy acetaldehyde (HPACET) and hydroperoxy acetone (HPAC). Although the yields of these compounds are small (<0.25 that of HPALD), the penultimate peroxy radicals (e.g., DHPALD-OO, Fig. 1) that form these compounds may, alternatively, undergo unimolecular isomerisation (see Fig. 1). Indeed, in the deuterated isoprene system, the apparent yield of HPACET and HPAC are greater, comparable to that of HPALD.
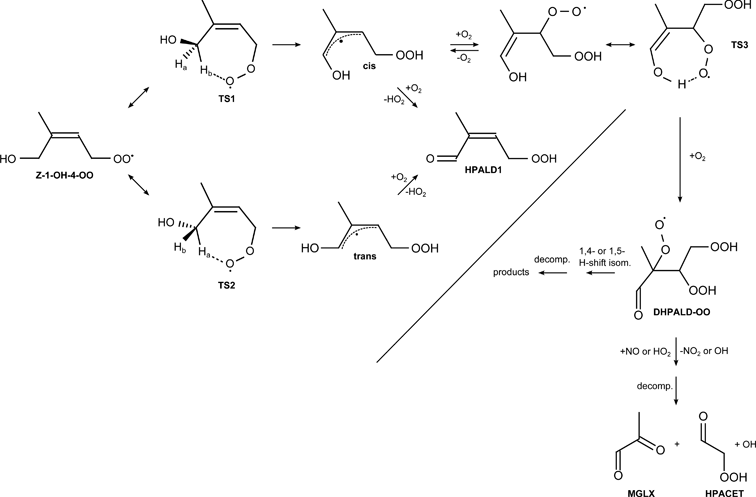
Fig. 1 Isomerisation pathways for the Z-1-OH-4-OO isoprene peroxyradical to form HPALD1 (as assumed in our paper), as well as an additional pathway to eventually yield hydroperoxy acetaldehyde (HPACET) which is achieved through a 1,6-enol-H-shift to the peroxy radical, similar to that studied by Peeters and Nguyen.5 In similar fashion, the 4-OH-1-OO isoprene peroxyradical (not shown) can degrade through analogous reactions to form HPALD2 and hydroperoxyacetone (HPAC). We observe HPACET and HPAC formation coincident with HPALD production in our chamber studies.
References:
1. D. Taraborrelli, M. G. Lawrence, J. N. Crowley, T. J. Dillon, S. Gromov, C. B. M. Gross, L. Vereecken and J. Lelieveld, Nat. Geosci., 2012, 5, 190–193.
2. O. Perrin, A. Heiss, K. Sahetchian, L. Kerhoas and J. Einhorn, Int. J. Chem. Kinet., 1998, 30, 875–887.
3. J. Peeters, T. L. Nguyen and L. Vereecken, Phys. Chem. Chem. Phys., 2009, 11, 5935–5939.
4. J. Peeters and J.-F. M¨uller, Phys. Chem. Chem. Phys., 2010, 12, 14227–14235.
5. J. Peeters and T. L. Nguyen, J. Phys. Chem. A, 2012, 116, 6134–6141
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article