Additions and corrections
Inkjet-printed graphene-PEDOT:PSS modified screen printed
carbon electrode for biochemical sensing
Chakrit Sriprachuabwong,a
Chanpen Karuwan,a Anurat
Wisitsorrat,a Ditsayut
Phokharatkul,a Tanom
Lomas,a Pornpimol
Sritongkhamb and Adisorn
Tuantranont*a
J. Mater. Chem., 2012, 22, 5478–5485 (DOI: 10.1039/c2jm14005e).
- In the published article, the image for Fig. 1 was inappropriate. The correct version of Fig. 1 is as follows (the caption is unchanged):
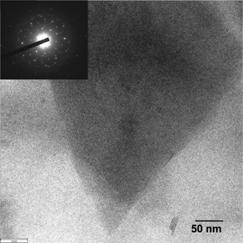
Fig. 1 Bright field TEM image of GP-PEDOT:PSS composite. Inset: selected area electron diffraction pattern of a region near an edge of a graphene sheet.
- In the published article, the image for Fig. 2 was inappropriate. The correct version of Fig. 2 is as follows (the caption is unchanged):
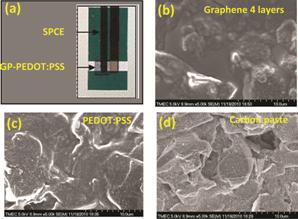
Fig. 2 (a) Typical photograph of inkjet-printed GP-PEDOT:PSS SPCE and SEM micrographs of (b) inkjet-printed GP-PEDOT:PSS SPCE, (c) inkjet-printed PEDOT:PSS SPCE and (d) SPCE.
- In the published article, the graph for Fig. 3 needs modification. The correct version of Fig. 3 is as follows (the caption is unchanged):
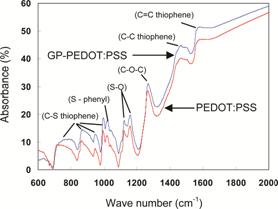
Fig. 3 FTIR spectra of GP-PEDOT:PSS and PEDOT layers on SPCEs.
- The Materials and methods section should read as follows:
Materials and
apparatus
All of chemicals used in this work were analytical grade. Standard solutions of H2O2, NADH and potassium hexacyanoferrate (K4Fe(CN)6) were purchased from Sigma (USA). Phosphate buffer (PB) solution (pH 6.8–7.4) was made from sodium dihydrogen phosphate (Fluka, Switzerland) and disodium hydrogen phosphate (Fluka). Potassium chloride (KCl) buffer solution (pH 7.0) was made from KCl powder (Fluka, Switzerland). The stock solutions of H2O2 (0.01 mol l−1), NADH (0.01 mol l−1) and K4Fe(CN)6 (0.01 mol l−1) were prepared by dissolving required amounts of chemicals in deionized distilled water. Graphite rods (1/4″ dia, Electron Microscopy Science) and a commercial PEDOT:PSS solution (clevios P jet N from HC Starck, USA) were used as starting materials for graphene synthesis by electrolytic exfoliation. An inkjet printer (Fujifilm Dimatix Materials Printer) was employed for printing of GP-PEDOT:PSS layers on SPCEs (in-house fabricated at King Mongkut University of Technology at Thonburi, Thailand). A potentiostat (μ-autolab Type III, Metrohm, Switzerland), a home-made 3 ml electrochemical cell made of acrylic, a GP-PEDOT:PSS working electrode, a platinum (Pt) wire counter electrode and a silver/silver chloride (Ag/AgCl) reference electrode were used for all CV measurements.
Preparation of
GP-PEDOT:PSS electrodes
GP-PEDOT:PSS electrode was prepared by one-step electrolytic exfoliation and ink-jet printing methods reported recently.35 In brief, a constant potential of 8 V was applied between two graphite rods immersed in PEDOT:PSS electrolyte for 5 hours and the supernatant portion of the dispersion subsequently centrifuged at 1200 rpm was decanted. The morphology and structure of graphene dispersed in the solution drop coated on a standard carbon/copper TEM grid were examined by transmission electron microscope (JEOL model JEM-2010). Graphene-PEDOT:PSS powder was extracted from the solution and its specific surface area was measured by nitrogen adsorption using Brunauer–Emmett–Teller (BET) analysis (Micromeritics model Tristar 3000). One to eight layers of GP-PEDOT:PSS material were inkjet-printed on SPCE over an area of 3 mm x 5 mm by the commercial material inkjet printer. The surface morphology, surface roughness and functional group of GP-PEDOT:PSS printed films were characterized using a scanning electron microscope (SEM, Hitachi model S-4700), a stylus surface profiler (Dektak Model 150) and a Fourier transform infrared spectrometer (FTIR, Perkin Elmer model spectrum spot light-300), respectively.
Electrochemical
measurement
The electrochemical characteristics of inkjet-printed GP-PEDOT:PSS modified SPCEs were measured by CV using the commercial electrochemical apparatus described above. H2O2, NADH and K4Fe(CN)6 solutions with different concentrations were then prepared for concentration study by proper dilution of the stock solutions in buffer solutions. The buffer solutions for H2O2, NADH and K4Fe(CN)6 are 0.1 M PB (pH 7.4), 0.1 M PB (pH 6.8) and 0.1 M KCl (pH 7.0), respectively. CV scans were performed at various scan rates for different concentrations of analytes. The selected voltage windows for H2O2, NADH and K4Fe(CN)6 were −0.4 to +1.2 V, 0 to 1.0 V and −0.2 to 0.8, respectively, because their redox peaks appeared within these potential ranges.
Additional reference
35 C. Karuwan, C. Sriprachuabwong, A. Wisitsoraat, D. Phokharatkul, P. Sritongkham, A. Tuantranont, Sens. Actuators, B, 2012, 161, 549.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Additions and corrections can be
viewed online by accessing the original article to which they apply.
Back to article