
Additions and corrections Metabolic response to low level toxicant exposure in a novel renal tubule epithelial cell system |
James Keith Ellis, Toby James Athersuch, Rachel Cavill, Robert Radford, Craig Slattery, Paul Jennings, Tara McMorrow, Michael P. Ryan, Timothy Mark David Ebbels and Hector Charles Keun
Mol. BioSyst., 2010, DOI: 10.1039/c0mb00146e. Amendment published 1st April 2011
Due to an unfortunate error during preparation of this manuscript, the labelling of several of the treatments was permuted and needs to be corrected throughout.
Where the following dosed compound is referred to, the following replacements should be applied:
NIF (Nifedipine) replaced with MON (Monuron)
KBrO3 (Potassium Bromate) replaced with NIF
MON replaced with KBrO3
OTA (Ochratoxin A) replaced with DIC (Diclofenac)
DIC replaced with OTA
Corrected versions of Figures 2–4 and Tables 1 and 2 (detailing all pertinent metabolic responses across all conditions) are included at the end of this document. The Supplementary Data table has also been corrected.
Irrespective of these corrections the central findings of the study as presented in the abstract remain accurate: namely that the metabolome of RPTEC/TERT1 cells can report on the effects of chemical exposure at low doses and that carcinogen treatment appeared to produce different and greater perturbations to metabolism compared to less toxic compounds. However some statements in the discussion, specifically those that refer to the metabolic effects we observed for particular treatments, are no longer valid and therefore we present the following additional corrections.
While originally stated to have few significant metabolic effects, the carcinogen KBrO3 in fact caused large changes to the metabolome, in particular to betaine and GPC levels. Broadly OTA and KBrO3 appeared to have similar effects on glycolysis and possibly mitochondrial metabolism. DIC appeared to cause several perturbations to metabolism but the pattern of change was dissimilar to other treatments e.g. DIC was the only compound to cause an apparent increase in betaine levels. Contrary to our original report the non-carcinogen NIF and the non-genotoxic carcinogen MON produced only minor metabolic effects.
Results Section
Paragraph 3
The sentence that reads: “As all the toxicants (MON, OTA, KBrO3) produced such effects at 72 h we focused on models from this time point to assist with the process of selecting key metabolites that could report on potential toxicity in this model (Figure 2A–D)” should be replaced with: “As all the toxic and pharmacologically active compounds (except MON) produced such effects at 72 h we focused on models from this time point to assist with the process of selecting key metabolites that could report on potential toxicity in this model (Figure 2A–D)”.
The sentence that reads: “OTA appeared to produce an opposite effect to MON, while the non-toxin DIC produced a broadly similar response in this component” should be replaced with: “DIC appeared to produce an opposite effect to KBrO3, while the toxin OTA produced a broadly similar response in this component.”
The sentence that reads: “Neither of the remaining non-toxins produced a consistent perturbation from control at this timepoint” should be replaced by: “Neither MON or MAN produced a consistent perturbation from control at this timepoint.”
Paragraph 5
The sentence that reads: “Interestingly, two carcinogens exhibited the opposite response, with major increases in GPC observed after exposure to MON (up to 1.76 fold, p=0.0008, 72h) and lesser increases observed in OTA exposed cells at earlier timepoints (up to 1.28 fold, p=0.04, 6h)” should be replaced with: “The genotoxic carcinogen KBrO3 and the analgesic/anti-inflammatory DIC exhibited the opposite response, with major increases in GPC observed after exposure to KBrO3 (up to 1.76 fold, p=0.0008, 72 h) and lesser increases observed in DIC exposed cells at earlier timepoints (up to 1.28 fold, p=0.04, 6 h)”.
Discussion
Paragraphs 2 to 7 should be replaced with:
“Ochratoxin A is a mycotoxin produced by some fungi and a common contaminant of foodstuffs. It has been classified as a carcinogen in the rodent kidney (reviewed in Mally et al., ref 24) but its mechanism of action is unclear as there is contrasting empirical evidence. However, it seems unlikely that it is directly genotoxic as there is little evidence for ochratoxin A-DNA adducts25-28 or 8-oxodG29,30 formation but it has been suggested that ochratoxin A mutagenesis is based on oxidative stress.31
A previous metabonomic study carried out in the rat suggested alteration of energy metabolism and mitochondrial function when the animals were dosed with 0-210 µg/kg body weight35 of OTA, where urinary citrate and 2-oxoglutarate levels were observed to decrease. In the present study OTA appears to affect the same metabolic pathways, where an increase in glycolysis without an increase in mitochondrial oxidative metabolism is observed. Additionally, urinary 5-oxoproline (glutathione depletion or oxidative stress) concentration was increased in the Sieber et al. study, which is an effect we do not observe in the RPTEC/TERT1 in vitro model. Of course, the rodent study by Sieber et al represents systemic changes at higher doses than the present study and OTA is also active in the liver.
DIC is a widely used analgesic and anti-inflammatory drug and can be hepatotoxic,37 with no genotoxic or carcinogenic action.38 DIC induced aerobic metabolism in our study where we observe a decrease in extracellular lactate with no accompanying uptake of glucose and increased excretion of pyruvate and 3-HV. The increase in intracellular GPC levels associated with DIC treatment at 6 and 24 h is suggestive of increased proliferation. KBrO3 treatment was more strongly associated with an increase in cellular proliferation than DIC, as a greater increase in intracellular GPC levels was observed. KBrO3 is a genotoxic carcinogen44 that causes the formation of 8-oxodG lesions in the rodent kidney.48 Treatment of the RPTEC/TERT1 cell line with KBrO3 caused multiple changes to the intracellular and media metabolite profile, suggesting that processes in addition to proliferation were affected. This is in contrast to a previous study,42 where it was observed that a 2 week dose of KBrO3 (100 and 200 ppm) caused no treatment related changes to the endogenous urinary metabolite profile. In the present study, KBrO3 and OTA both had a negative effect on mitochondrial metabolism. Although KBrO3 and OTA are considered as different types of carcinogen (KBrO3 is genotoxic45,46 and OTA is probably non-genotoxic24) both show an increase in glycolysis without an increase in mitochondrial oxidative metabolism. Considered together, these changes and the decreased amounts of betaine (both compounds) and choline (KBrO3 only) indicate that mitochondrial metabolism may be perturbed by these treatments.
DIC may also be altering the transportation of lactate and pyruvate across cellular membranes. In a primary epithelial cell line39 (human retinal origin) and immortalised cell line40 (human corneal origin), DIC exposure causes activity changes in the monocarboxylate transporters (SMCT1 and MCT1/MCT4). These effects are of course observed in a very specific cell type but SMCT1 and SMCT2 are expressed in the kidney41 and are involved in lactate and pyruvate transport.
A minor increase was detected in extracellular 5-oxoproline levels after 24 h of exposure to MON and NIF. NIF is a dihydropyridine calcium channel blocker and is used as a antihypertensive drug.43 MON is a herbicide that causes renal and hepatic carcinogenesis in the male rat through a non-genotoxic mechanism of action.36 5-oxoproline is an intermediate in the γ-glutamyl cycle of glutathione metabolism and a reduction of GSH synthesis can result in a raised level of 5-oxoproline.22 5-oxoprolinuria has been observed in rats that have been dosed with chemicals that induce glutathione or cysteine depletion.19,47 NIF has been shown to be photosensitive and undergo oxidation when exposed to light49-51 and could cause a transient increase in GSH turnover, whilst not affecting the concentration of the glutathione pool, and increase oxidative stress.”
Corrected Tables
Table 1. Major Intracellular Metabolite Changes (Fold change relative to control).
 |
Metabolites shown in the above table exhibited major changes (>1.5 fold) in intracellular levels for at least one experimental condition. Control = 1. GPC = glycerophosphocholine.
Table 2. Major Extracellular metabolite changes (Fold change relative to control) in the media at 72 hours.
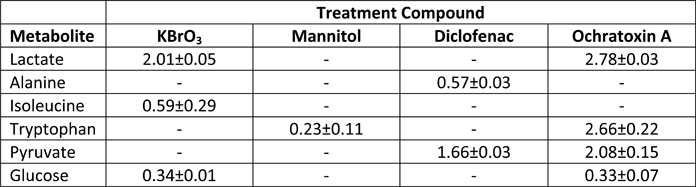 |
Metabolites shown in the above table exhibited major differences (>1.5 fold) in extracellular concentration compared to controls at the end of the experiment (72 h) for at least one condition. Control = 1.
Corrected Figures
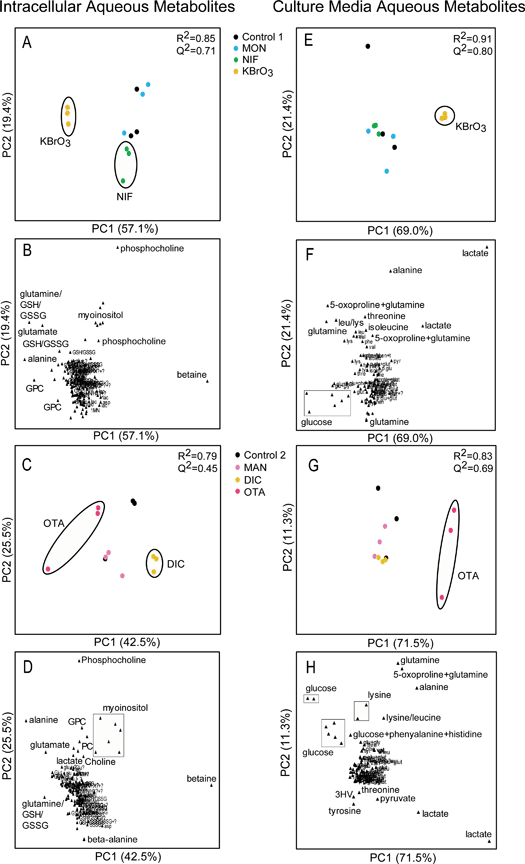 |
Figure 2. The Response of the RPTEC/TERT1 Cell Line to Specific Toxins at 72 h in both the aqueous cellular extract and the culture medium. Principal component analysis (PCA) was applied to the intracellular and media metabolic profiles to assess treatment related effects. The reduced resolution data (Intracellular = 166 data points/media = 96 data points of varying ppm width) were Pareto scaled in all models. NIF – Nifedipine, KBrO3 – Potassium Bromate, MON – Monuron, MAN – D-Mannitol, OTA – Ochratoxin A, DIC – Sodium Diclofenac. PCA scores plots (A, E, C and G) and accompanying loadings plots (B, F, D and H ). PC = Principal component. G & H = Phenol resonances removed from analysis.
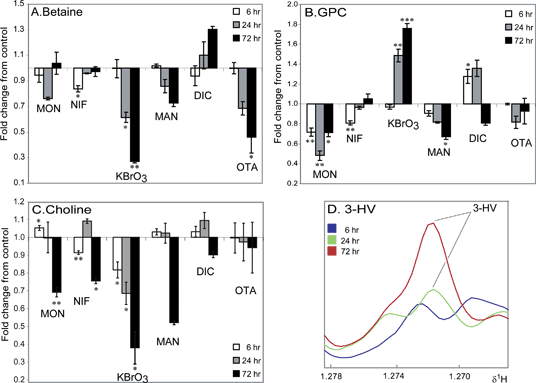 |
Figure 3. Effect of Compound Treatment on the Concentration of Three Intracellular Metabolites and the Effect of Sodium Diclofenac Treatment on Extracellular 3-HV Concentration. (A): Change in relative concentration (Fold change relative to control) of intracellular betaine levels in all six treatment groups. (B): Change in relative concentration (Fold change relative to control) of intracellular GPC levels in all six treatment groups. (C): Change in relative concentration (Fold change relative to control) of intracellular choline levels in all six treatment groups. (D): Averaged 1H CPMG spin-echo NMR spectra (n = 3 per timepoint) showing the increase in 3-HV concentration in the media of DIC treated RPTEC/TERT1 cells. 3-HV = 3-hydroxy-isovalerate. NIF – Nifedipine, KBrO3 – Potassium Bromate, MON – Monuron, MAN – D-Mannitol, OTA – Ochratoxin A, DIC – Sodium Diclofenac. Significance compared to control assessed by Welch’s t-test. NIF, KBrO3 and MON compared to control 1 and MAN, OTA and DIC compared to control 2. Control = 1. Significance marked as: * p<0.05, ** p<0.01, *** p<0.001. Significance values indicated are without multiple testing correction. Upon Bonferroni correction fold changes with a p<0.001 remain valid (q<0.05).
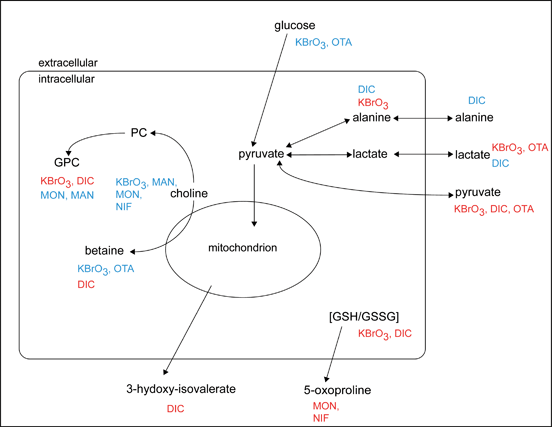 |
Figure 4. Overview of the intracellular and extracellular metabolic changes in the RPTEC/TERT1 cell line associated with compound exposure. Red indicates that the treatment compound increases the metabolite. Blue indicates that the treatment compound decreases the metabolite.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.