The first solid-state structural characterisation of a thioether I-Cl adduct is presented along with new much improved theoretical calculations for the accurate modelling of thioether interhalogen adducts.
3D Coordinates in the form of MDL Molfiles and CML files, for all geometries and associated energies (Hartree). Calculations performed using Gaussian 98 using General basis sets1 including the the MIDI! basis set2 and pVTZ basis set3. Note: The G03 program uses an SVD algorithm to retain as many functions a possible when doing the test for linear dependence in basis sets. This results in retention of more basis functions then G98, and hence slightly lower energies. Retention of all basis functions can be forced using the keyword IOP(3/32=2). The KMLYP method4, CCSD method5 and CPCM (polarizable conductor calculation model)6 were used.
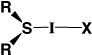
| Table 1. Energies and Geometries for X=F,Cl,Br,I. | |||
|---|---|---|---|
| X | Energy (Hartree), Method/basis set | S...I distance, Å (experimental values) | I-X distance, Å (experimental values) |
| F | -7497.44379, B3LYP/6-311G(d,p) | 2.857 | 2.029 |
| F | -7498.36888, B3LYP/pVTZ | 2.796 | 2.014 |
| F | -7495.00211, KMLYP/6-311G(d,p) | 2.754 | 1.973 |
| F | -7495.95301, KMLYP/pVTZa | 2.713 | 1.948 |
| F | -7495.95573, KMLYP(CPCM)/pVTZ | 2.592 | 2.019 |
| Cl | -7857.83074, B3LYP/6-311G(d,p) | 2.908 (2.575, 2.556) | 2.491 (2.558, 2.604) |
| Cl | -7855.16216, KMLYP/6-311G(d,p) | 2.827 | 2.410 |
| Cl | -7853.94815, CCSD/6-311G(d,p) | 3.047 | 2.428 |
| Cl | -7823.80997, B3LYP/MIDI! | 2.836 | 2.453 |
| Cl | -7821.18482, KMLYP/MIDI! | 2.778 | 2.377 |
| Cl | -7819.68286, CCSD/MIDI! | 2.971 | 2.388 |
| Cl | -7821.18715, KMLYP(CPCM)/MIDI! | 2.608 | 2.472 |
| Cl | -7858.74220, B3LYP/pVTZ | 2.881 | 2.470 |
| Cl | -7856.10083, KMLYP/pVTZb | 2.805 | 2.394 |
| Cl | -7856.10101, KMLYP(CPCM)/pVTZ | 2.623 | 2.493 |
| Clc | -8775.69715, KMLYP/pVTZ | 2.860 | 2.359, 2.466 |
| Cld | -15712.2081, KMLYP/pVTZ | 2.805 | 2.397, 3.715 |
| Br | -9972.67817, B3LYP/pVTZ | 2.924 (2.615, 2.620, 2.679, 2.617, 2.687) | 2.617 (2.690, 2.694, 2.654, 2.705, 2.645) |
| Br | -9971.76233, B3LYP/6-311G(d,p) | 2.973 | 2.624 |
| Br | -9968.44731, KMLYP/6-311G(d,p) | 2.894 | 2.538 |
| Br | -9969.38767, KMLYP/pVTZe | 2.849 | 2.535 |
| Br | -9969.38702, KMLYP(CPCM)/pVTZ | 2.648 | 2.634 |
| I | -14317.15297, B3LYP/6-311G(d,p) | 3.059 (2.886, 2.812) | 2.824 (2.781, 2.803) |
| I | -14318.98171, B3LYP/pVTZ | 3.025 | 2.807 |
| I | -14313.16245, KMLYP/6-311G(d,p) | 2.996 | 2.729 |
| I | -14315.03279, KMLYP/pVTZf | 2.962 | 2.715 |
| I | -14315.03695, KMLYP(CPCM)/pVTZ | 2.726 | 2.804 |
a ΔG298dissociation 8.8 kcal/mol. b ΔG298dissociation 3.9 kcal/mol. c For ICl3 system. d Dimer. e ΔG298dissociation 2.7 kcal/mol. f ΔG298dissociation 0.2 kcal/mol.
| Table 2. NBO Analysis (KMLYP/pVTZ) | ||||
|---|---|---|---|---|
| Natural property | F | Cl | Br | I |
| Charge on X | -0.66 | -0.41 | -0.30 | -0.15 |
| Charge on I | +0.43 | +0.18 | +0.10 | -0.02 |
| Slp to I-X σ* Interaction energy | 63.5 | 50.5 | 45.7 | 30.8 |
| Slp occupancy | 1.71 | 1.73 | 1.74 | 1.78 |
| X-Iσ* occupancy | 0.24 | 0.22 | 0.21 | 0.17 |
| Table 3. Normal coordinate analysis for X=Cl,Br (KMLYP//pVTZ). | |
|---|---|
| Cl-I stretch 346 | S-I stretch 125 |
| Br-I stretch 242 | S-I stretch 113 |
| Table 4. Crystallographic Coordinates and Data for Molecule 1 in CML Format |
|---|
| INChI: 1.12Beta/C14H15ClIS/c15-16-17(11-13-7-3-1-4-8-13)12-14-9-5-2-6-10-14/h1-10H,11-12H2,16H |
Ab initio calculations were performed using the Gaussian 98 and Gaussian 03 programs.7 The KMLYP procedure4 was invoked using the keywords iop(3/76=1000005570) iop(3/77=0000004430) and iop(3/78=0448010000) (Gaussian 03). Full geometry optmisation was followed by zero point and entropy energy correction using unscaled calculated frequencies. NBO analysis followed Weihold's protocols.8