
Scheme 1. R=R' = (a) NO2; (b) NO; (c) CHO; (d) CHS; (e) CF; (f) CF3
Carlota Conesa and Henry S. Rzepa*
Department of Chemistry, Imperial college of Science, Technology and Medicine,
South Kensington, London SW7 2AY, UK


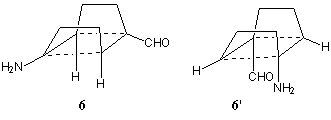
Results and Discussion. Initial studies using the rapid PM3 method led us to focus on the R and R' positions as the most sensitive location for substituents. Initially, difunctionalisation with the electron push-pull substituents R=CHO, R'=NH2 was studied, but this resulted in little energy stabilisation relative to the unsubstituted systems. The stationary points that were located revealed three imaginary frequencies for both possible geometric isomers; 6: DHPM3=137.08 kcal.mol-1 n = 1054.0i, 952.6i, 325.3i cm-1; 6': DH PM3=134.20 kcal.mol-1 n = 902.6i, 617.3i, 490.5i cm-1.
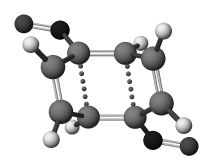
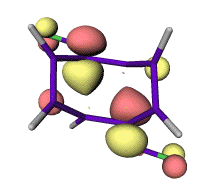
A significant difference was observed when both R and R' were selected to be an electron-withdrawing groups. In this case, the computed barriers to reaction decrease significantly and all the studied structures 6a-f were now found to be genuine transition states at PM3 level. In each case, only isomer 6 rather than 6' could be located. The form of the now single imaginary vibrational mode most resembles a p2s+p2s cyclo-elimination rather than two synchronous conrotatory ring openings, but with a distinct trapezoidal component to the geometry (Figure 1) which still clearly corresponds to suprafacial bond cleavage/formation across the two alkene components. This induced trapezoidal mode is the principal geometric feature which allows the reaction to access a genuine saddle point on the closed shell potential surface, but one quite different in character to the alternative Woodward-Hoffmann geometrical process involving a single antarafacial component on one alkene. When the anti isomer 12 (Scheme 1) was studied, analogous results were obtained, leading to the product corresponding to the isomeric cyclooctatetraene 11.
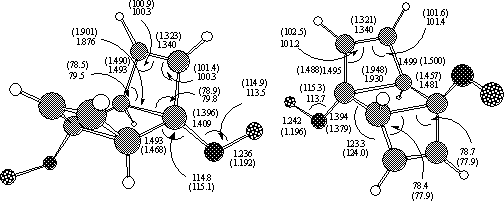
These PM3 transition structures were then re-optimised with ab initio methods. At the RHF/6-31G(d) level, 6 and 12 are computed as a second order saddle points, with the genuine transition states (2 and 8) corresponding to the 4p-conrotatory opening of a side ring being lower in energy in all instances. With re-optimisation at the B3LYP density functional theory level, structures 6 and 12 again show only one imaginary frequency, except in specific instances of 6a (R=NO2) and 6f (R=CF3) where imaginary rotational modes of the substituent could not be eliminated. The geometry for one example is shown in Figure 1. The trapezoidal angle ranges from 105.7° to 112.7°, with relatively little correlation with substituent.
Analysis of the frontier molecular orbital at the transition state in these
systems shows greatly reduced electronic density on the carbon atom in b
position to the substituent (Figure 2). Each forming s bond is therefore
associated predominantly with p electrons deriving from only one of the two
alkene components, a process facilitated by the trapezoidal distortion of the
geometry. This allows the creation of transition state saddle points in the
potential surface to allow a ground state thermal reaction to proceed.
The energies computed for 6 are comparable to those obtained for
transition states 2 (Table 1). Distinct geometries corresponding to
2b, 2d and 2e could not be located in the potential
surface at the B3LYP level, with geometry optimisation in these cases
converging to the corresponding synchronous structure 6. In contrast,
the energy values for the transition states corresponding to the
cycloelimination of the trans- isomer 12 are higher in energy
than these obtained for the ring opening transformations 8 in all the
examples except 8e (Table 2). This does imply that the kinetic
behaviour of the syn series (proceeding via 6) might be
quite distinct from the anti series, which is still predicted to proceed
via 8 and not 12. Overall, the low energy barriers
computed for 6d and 6e in particular suggest that the synchronous
reaction pathway for these two systems in particular should be readily
accessible under thermal conditions, and that experimental verification could
be sought for this system.
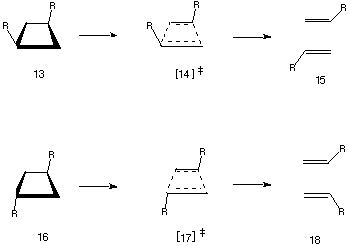
In light of these results, we decided to also study the potential surfaces for
the parent s2s+s2s cycloelimination
of the corresponding substituted cyclobutanes 13 and 16 (Table
3). A biradical mechanism for the
p2s+p2s cycloaddition/elimination
of ethene had previously been computed for this process by Bernardi et
al.[6] and by Olivella.[7] Antarafacial geometries
(p2s+p2a) were found by Bernardi
et al to correspond to second order saddle points at MCSCF levels of
theory, distorting to biradical-like transition states. Higher cycloaddition
homologues such as p4s+p4a and
p6s+p6a cycloadditions[8] were similarly found to be second-order saddle
points at RHF levels of theory. The characteristic feature of all these
previous studies is that no genuine transition states could be located at the
single-determinantal closed shell RHF method, and only the use of
multi-configuration open-shell (MCSCF) methods led to any first-order saddle
points. To our knowledge an alternative trapezoidal pathway for cycloaddition
reactions has not hitherto been discussed or characterised.
In our case, the single determinantal RHF method applied to the structures
14a-f and 17a-f did indeed result in characterisable transition
states (Table 3), the most noticeable features of which are again a strongly
trapezoidal C2h geometry, but in this case associated also with
thermally unrealistic high energy barriers. These trapezoidal characteristic
angles (ranging from 106.0° to 115.8°) are more pronounced for
14a-f and 17a-f than for 6a-f and 12a-f because of
the absence of the additional geometrical constraints induced by the additional
rings in the latter. Nevertheless, these results do establish that the
qualitative change in the potential energy surface for the
p2s+p2s cycloaddition/elimination
reaction was not fundamentally due to the characteristics present in 6
or 12, such as ring strain or torquoselectivity effects on the
conrotatory electrocyclic ring opening.[9]
Rather, it is the distortions in the wavefunctions at the transition state due
to the presence of the electron withdrawing substituents that creates a
thermally allowed reaction mode.
Conclusions. We have established that at PM3 or B3LYP/6-31G(d) levels
of theory, the potential energy surface for a simple pericyclic reaction such
as a p2s+p2s
cycloaddition/elimination can be qualitatively modified by pairs of electron
withdrawing substituents, to allow a geometric alternative to the
Woodward-Hoffmann antarafacial distortion required for a thermal reaction. Such
a trapezoidal distortion allows a fully synchronous transition state 6
for the transformation of species 1 to 5 to become the predicted
favoured route with substituents such as R=NO, CHS or CF
(carbene). We are currently investigating whether a similar re-engineering of
the potential energy surface might be possible for other related examples of
pericyclic reactions.
|
|
PM3 |
?i |
RHF/6-31G(d) |
?i |
B3LYP/6-31G(d) |
?i |
|
6a |
(32.13) |
-1462.4, 35.4 |
(44.76) |
-729.9, -156.4 |
(29.04) |
-581.0, -57.4 |
|
2a |
(30.70) |
-404.0, 24.0 |
(39.81) |
73.9 |
(26.13) |
33.4 |
|
6b |
(34.77) |
-1726.0, 55.4 |
(40.11) |
-177.0 |
(22.15) |
71.9 |
|
2b |
(30.68) |
-465.4, 50.2 |
(38.17) |
89.6 |
a |
|
|
6c |
(37.59) |
-1931.4, 56.0 |
(38.94) |
-157.0 |
(29.34) |
50.5 |
|
2c |
(31.71) |
-502.1, 67.7 |
(43.02) |
77.2 |
(29.33) |
75.1 |
|
6d |
(30.98) |
-1603.0, 38.2 |
(35.47) |
-184.2 |
(20.38) |
59.7 |
|
2d |
(27.20) |
-378.3, 73.1 |
(34.66) |
55.8 |
a |
|
|
6e |
(20.81) |
-1114.4, 65.9 |
(26.19) |
-94.7 |
(16.64) |
77.5 |
|
2e |
a |
|
a |
|
a |
|
|
6f |
(39.55) |
-2092.0, 11.5 |
(58.82) |
-188.8 |
(41.81) |
-111.8 |
|
2f |
(32.10) |
-505.8, 22.4 |
(46.70) |
42.49 |
(25.38) |
a. Structure 2 converges to 6 on optimisation.
Table 2. Energies (kcal/mol for PM3, otherwise Hartree), Barriers (kcal mol-1, in parentheses) and Imaginary normal modes (cm-1) for stationary point structures in the anti series 12a-f and 8a-f.
|
|
PM3 |
?i |
RHF/6-31G(d) |
?i |
B3LYP/6-31G(d) |
?i |
|
12a |
(41.32) |
-1536.7, 12.1 |
(67.32) |
-199.5 |
(44.92) |
-50.0 |
|
8a |
(24.01) |
-32.3, 34.5 |
(48.64) |
53.6 |
(31.71) |
51.9 |
|
12b |
(42.58) |
-2192.0, -24.0 |
(56.6) |
-227.4 |
(31.28) |
64.8 |
|
8b |
(29.00) |
-614.9, 36.3 |
(45.96) |
82.4 |
(27.31) |
84.1 |
|
12c |
(37.59) |
-1931.4, 56.0 |
(62.89) |
-230.2 |
(41.85) |
13.2 |
|
8c |
(34.56) |
-1864.7, -52.9 |
(42.83) |
82.1 |
(29.24) |
79.6 |
|
12d |
(31.71) |
-505.4, 36.4 |
(49.3) |
-265.2 |
(29.29) |
38.7 |
|
8d |
(25.92) |
-399.9, 36.0 |
(43.29) |
55.4 |
(26.56) |
-220.4 , 50.4 |
|
12e |
(25.22) |
-1185.3, 39.5 |
(37.99) |
-166.5 |
(22.88) |
65.0 |
|
8e |
Not optimizable |
|
(36.51) |
53.5 |
a |
|
|
12f |
(46.94) |
-2731.2, 20.7 |
(78.12) |
-291.2 |
(56.64) |
-88.5 |
|
8f |
(31.99) |
-491.8, 14.1 |
(51.50) |
38.2 |
(36.61) |
35.8 |
a. Structure 8 converges to 12 on optimisation.
Table 3. Energies (barriers, kcal mol-1) and Imaginary normal modes (cm-1) for the stationary point structures 14a-e and 17a-e.
|
|
PM3 |
?i |
RHF/6-31G(d) |
?i |
B3LYP/6-31G(d) |
|
|
14a |
(81.48) |
-2736.1 |
(98.75) |
68.15 |
(78.19) |
68.6 |
|
14b |
(87.07) |
-3699.3, 46.2 |
(91.42) |
77.2 |
(62.38) |
81.1 |
|
14c |
(90.26) |
-7597.7, 64.0 |
(93.80) |
63.7 |
(70.87) |
64.0 |
|
14d |
(81.09) |
-3975.2, 34.1 |
(82.02) |
42.9 |
(58.85) |
47.4 |
|
14e |
(71.34) |
-1621.7, 64.8 |
(67.68) |
86.8 |
(46.89) |
88.4 |
|
17a |
(80.60) |
-2602.0, 37.6 |
(94.18) |
67.4 |
(73.94) |
63.7 |
|
17b |
(86.90) |
-3687.4, 49.4 |
(88.93) |
73.8 |
(61.73) |
70.0 |
|
17c |
(90.23) |
-7870.0, 49.6 |
(94.92) |
65.3 |
(72.37) |
57.0 |
|
17d |
(81.30) |
-4133.4, 36.4 |
(80.86) |
42.0 |
(58.76) |
34.5 |
|
17e |
(70.53) |
-1670.8, 51.7 |
(67.07) |
62.8 |
68.9 |