Additions and corrections
Role of charge transfer states into the formation of cyclobutane pyrimidine dimers in DNA
Wook Lee and Spiridoula Matsika*
Faraday Discuss., 2019, DOI: 10.1039/C8FD00184G. Amendment published 1st July 2019.
After this paper (Role of charge transfer states into the formation of cyclobutene pyrimidine dimers in DNA) was accepted we found out that the partial charges we had used for methylated cytosine (5mC) are extremely sensitive to the details of obtaining them. In order to be able to compare directly cytosine and methylated cytosine the charges should be as similar as possible. So, we recalculated the charges on 5mC in the exact same protocol as the ones for cytosine. The details of how this was done and how the results changed are outlined below. We want to emphasize, however, that we see this as a cautionary tale. The partial charges are extremely sensitive to details, such as the number of grid points, and this is unsettling. So, one has to be very careful in obtaining these charges, and in our opinion benchmarks have to be made to decide which charges are best. Ideally, quantum mechanical calculations should be carried out to decide which answer is closer to reality. Furthermore, when charge transfer states are calculated, the charges can change the energies significantly, so even further caution is needed.
The RESP procedure we carried out to obtain the 5mC charges has some small deviations from the one originally used to obtain AMBER force field charges for nucleobases. The first deviation is that we used far more grid points than the default value used in Gaussian for fitting point charges (thinking mistakenly that this would make it more accurate), and the second is that the fragment we used for generating RESP charges is different from the one used for generating standard AMBER force field.1 In standard AMBER force field, the whole nucleoside was included in the calculations when atomic partial charges were derived, while we only included the base and part of a sugar ring. The main difference between the charges in C and 5 mC that affects our results is the charge at C8 atom, which is at the boundary between the QM and MM region (see Figure 1). This charge was rather large in our original calculations. After correcting for the two factors described above, we could obtain a small positive charge of C8 atom as shown in Table 1.
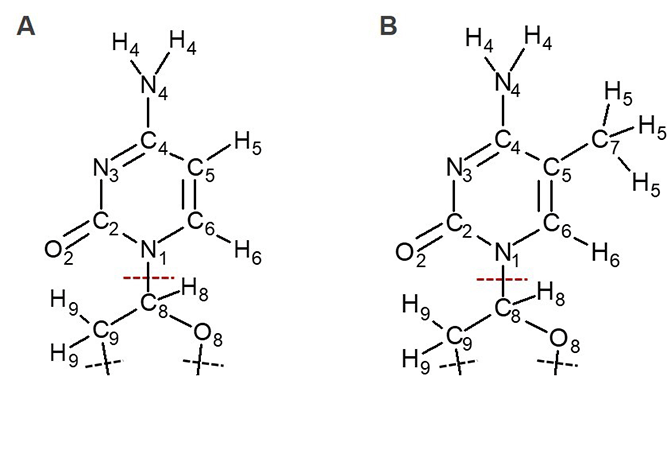
|
Table 1. Atomic partial
charges of cytosine from standard AMBER force fields (ff99) and the RESP
charges of 5mC from the original paper and new calculations |
|||
|
Cytosine
(AMBER ff99) |
Original
charges of 5 mC |
New
charges of 5 mC |
|
|
N1
|
-0.0339
|
-0.3408
|
-0.0398
|
|
C2
|
0.7959
|
0.8749
|
0.7063
|
|
O2
|
-0.6548
|
-0.6594
|
-0.6288
|
|
N3
|
-0.7748
|
-0.7944
|
-0.6882
|
|
C4
|
0.8439
|
0.7942
|
0.6341
|
|
N4
|
-0.9773
|
-0.9514
|
-0.8770
|
|
H4
|
0.4314
|
0.4159
|
0.3943
|
|
C5
|
-0.5222
|
-0.1936
|
-0.1182
|
|
H5
|
0.1863
|
0.1033
|
0.0767
|
|
C6
|
-0.0183
|
-0.0126
|
-0.1343
|
|
H6
|
0.2293
|
0.1972
|
0.2350
|
|
C8
|
-0.0116
|
0.5571
|
0.0484
|
|
H8
|
0.1963
|
0.0421
|
0.1963
|
|
O8
|
-0.3691
|
-0.4738
|
-0.3691
|
|
C9
|
-0.0854
|
-0.1901
|
-0.0854
|
|
H9
|
0.0718
|
0.0718
|
0.0718
|
|
C7
|
-0.3239
|
-0.2309
|
|
With these new charges of 5mC, we re-computed the vertical excitation energy of charge transfer states for the sequences involving 5mC, GmCT and TmCG. Figures 4 and 5 in the original paper need to be updated with the new energies. The updated figures are shown below.
The main effect of the new charges is the description of methylation effects in Section 3.2.3 of the manuscript. According to the new results methylation does not have a pronounced effect on the charge transfer states. In fact, methylation causes a small effect in the energies of charge transfer states, which follows the trends of the distances between donor and acceptor. The main idea described in Section 3.3.3, i.e. that the electrostatic interactions with the surroundings will affect the charge transfer states, should still be valid. But there is no direct evidence of that in our study. This is shown in the updated section of Table 2 below. This shows that the energy of charge transfer states is not dramatically different between QM and QM/MM calculations, indicating that the surroundings do not play such a big role as we originally thought.
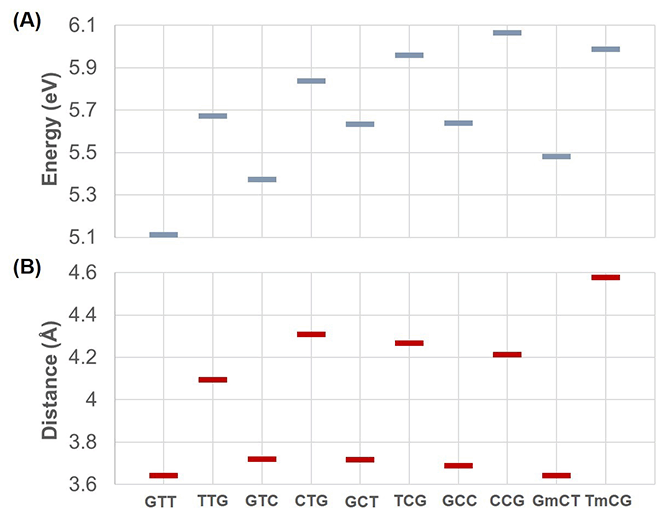
Updated Figure 4: (A) Average charge transfer energies obtained from 20 snapshots and (B) average distances between two bases involved in charge transfer obtained from 200 snapshots for all sequences. The only different results here compared to the original figure are in part (A) the last two energies (corresponding to GmCT and TmCG).
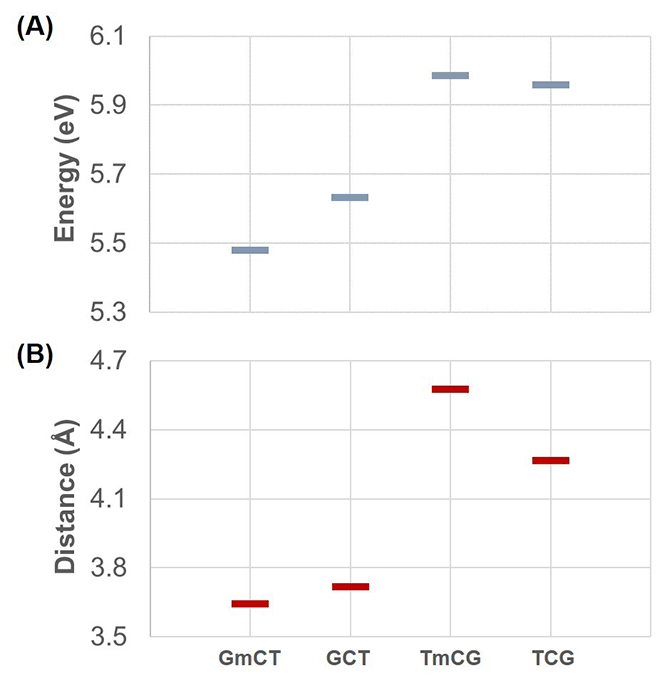
Updated
|
Updated Table 2: Energies
of charge transfer states (in eV) for a representative snapshot calculated
using the whole model system (QM and MM) and only the QM region. The energy
difference ΔE between the two previously listed sequences is also shown. |
||
|
QM/MM |
QM |
|
|
GmCT |
5.452 |
5.384 |
|
GCT |
5.649 |
5.231 |
|
ΔEGCT-GmCT |
0.197 |
-0.153 |
|
TmCG |
5.978 |
5.920 |
|
TCG |
5.942 |
5.803 |
|
ΔETCG-TmCG |
-0.036 |
-0.117 |
Reference
1 P. Cieplak, W. D. Cornell, C. Bayly, P. A. Kollman, J. Comput. Chem. 1995, 16, 1357–1377.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article