Additions and corrections
Enantioselective epoxidation of trans-disubstituted alkenes by D2-symmetric chiral dioxoruthenium(VI) porphyrins
Rui Zhang, Wing-Yui Yu, Tat-Shing Lai and Chi-Ming Che
Chem. Commun., 1999, 409.
The FABMS data on page 410 for 1a are incorrect. The correct data should be: FABMS m/z: 1379 (M+, 18%), 1363 (M+ - O, 30%), 1347 (M+ - 2O, 100%).
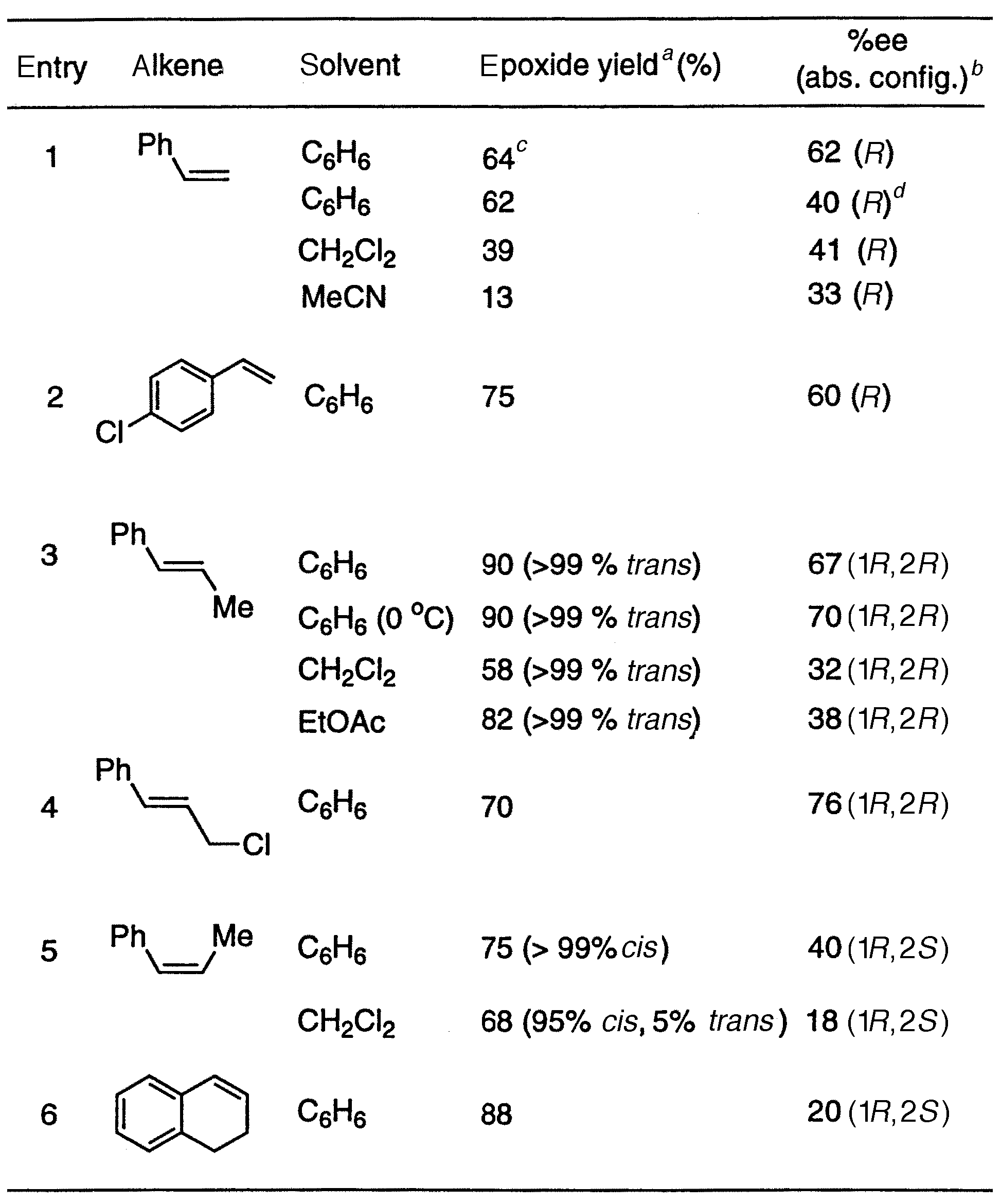
Also, in Table 1, the absolute configurations for entries 3-6 are incorrect; the correct configuration should be 1R,2R for entries 3 and 4, and 1R,2S for entries 5 and 6. The corrected table is as follows:
Table 1 Stoichiometric epoxidation of some aromatic alkenes by [RuVI(L1*)O2] (1a)

Reaction conditions: To a degassed benzene solution (1 cm3) containing alkene (1 mmol) and pyrazole (0.3 mmol) was added the dioxoruthenium(VI) complex (0.015-0.03 mmol) under an argon atmosphere. The reaction mixture was stirred at room temperature (or otherwise noted) for 12 h, and the aliquot was analyzed by gas chromatography for product identification and quantification. a Yields are based on the amount of the oxidant used. b Enantiopurities (%ee) of the epoxides were determined by GC equipped with a chiral capillary column (J & W Scientific cyclodex-B or G-TA). Absolute configuration was determined by comparing with authentic chiral samples. c Benzaldehyde (27%) and phenylacetaldehyde (12%) were also detected. d Reaction was carried out without pyrazole.