Additions and corrections
Synthesis and structure of the [MnIV(biguanide)3]4+ ion: the simplest source for water-stable manganese(IV)
Gopal Das, Parimal K. Bharadwaj, Debjani Ghosh, Beauty Chaudhuri and Rupendranath Bannerjee
Chem. Commun., 2001, 323 (DOI: 10.1039/b007013k). Amendment published 24th September 2002
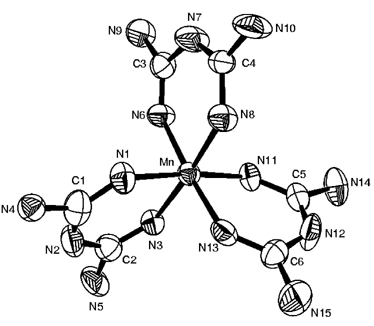
In this communication, the crystal structure was wrongly reported in the non-centrosymmetric space group Ia with two formula units of [Mn(bigH)3]·3NO3·½SO4·3H2O present in the asymmetric unit (hence, Z = 8). The correct space group for this structure is, in fact, the centrosymmetric I2/a with only one formula unit in the asymmetric unit (again, Z = 8). The structure in the correct space group (Fig. 1 below) does not show any unusual thermal ellipsoids unlike Fig. 1 in the original paper. The coordination geometry around the metal ion is quite regular and is not axially distorted. There are no significant N···N non-bonded interactions and Fig. 2 in the original paper should be discounted. The crystal packing is, in fact, dominated by a number of N–H···O hydrogen bonds of normal geometry. This crystal and subsequently all other crystals of the compound prepared at different times, did not diffract well and one of the nitrate anions was severely disordered. The X-ray data alone cannot conclusively establish that this is a Mn(IV) complex, although the structure taken together with chemical and spectroscopic results do provide cogent evidence. A new CIF file, CCDC 182/1886, has been deposited at the Cambridge Crystallographic Data Centre and can be viewed at http://www.rsc.org/suppdata/cc/b0/b007013k/

Fig. 1 ORTEP plot and labeling for [Mn(bigH)3]4+. For clarity the hydrogen atoms are not shown. Selected bond distances (Å) and angles (°): Mn–N(8) 1.943(7), Mn–N(11) 1.935(6), Mn–N(13) 1.923(7), Mn–N(1) 1.934(7), Mn–N(3) 1.928(6), Mn–N(6) 1.930(7), N(8)–Mn–N(11) 92.5(3), N(8)–Mn–N(13) 90.1(3) , N(11)–Mn–N(13) 86.4(3), N(8)–Mn–N(1) 90.1(3), N(11)–Mn–N(1) 175.5(3), N(13)–Mn–N(1) 90.0(3), N(8)–Mn–N(3) 175.4(3), N(11)–Mn–N(3) 90.6(3), N(13)–Mn–N(3) 93.5(3), N(1)–Mn–N(3) 87.0(3), N(8)–Mn–N(6) 87.0(3), N(11)–Mn–N(6) 90.4(3), N(13)–Mn–N(6) 175.6(3), N(1)–Mn–N(6) 93.4(3), N(3)–Mn–N(6) 89.6(3).
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.