Additions and corrections
Synthesis of the dicationic RuIV amido complex [TpRu(CO)(PPh3)(NHPh)][OTf]2 (Tp = hydridotris(pyrazolyl)borate; OTf = trifluoromethanesulfonate) and deprotonation to form an octahedral and d4 imido complex: computational study of RuIV–imido bonding
K. N. Jayaprakash, Aaron M. Gillepsie, T. Brent Gunnoe and David P. White
Chem. Commun., 2002, 372 (DOI: 10.1039/b110999e). Amendment published 16th July 2002
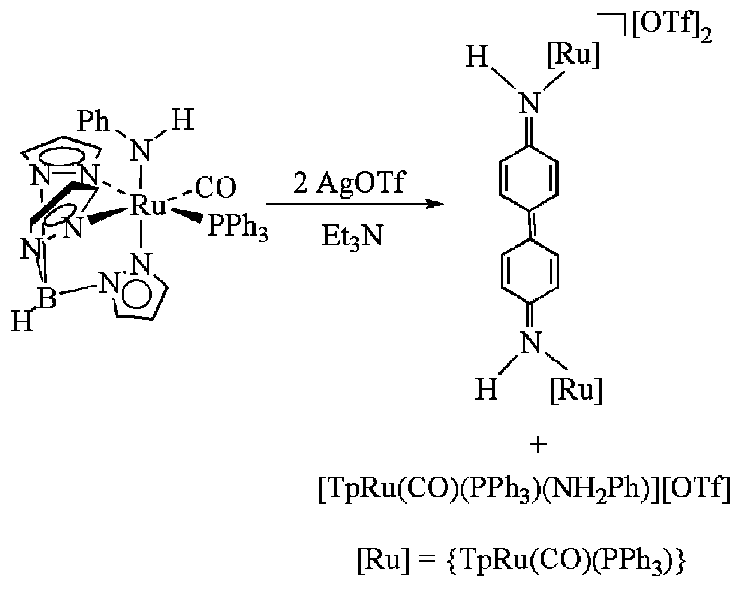
Based on 1H, 13C and 31P NMR spectroscopy, IR spectroscopy, elemental analysis, isotopic labeling studies and deuterium exchange with CD3OD, the isolated products from the reaction of TpRu(CO)(PPh3)(NHPh) with two equivalents of AgOTf and Et3N {followed by anion metathesis with NaBAr'4 (Ar' = 3,5-(CF3)2C6H3)} was reported to be the Ru(IV) amido complexes [TpRu(CO)(PPh3)(NHPh)[X]2 (X = OTf or BAr'4). The aromatic region in the 1H NMR spectra of these complexes is complicated due to multiple overlapping resonances. By extension of this work to include TpRuL2(NHPh) (L = CO, P(OMe)3 or PMe3) complexes we have been able to definitively determine that the reaction product is not the monomeric Ru(IV) amido complex but rather the binuclear complex [TpRu(CO)(PPh3)NH(C6H4-)]2[OTf]2 (see Scheme below). The use of the non-aromatic ligands CO, P(OMe)3 and PMe3 results in a less cluttered aromatic region of the 1H NMR and reveals that the para C–H bond has been broken. In addition, a solid-state structure of [TpRu{P(OMe)3}2NH(C6H4-)]2[OTf]2 has confirmed the characterization. The physical characterization data for [TpRu(CO)(PPh3)(NHPh)][OTf]2 are given below. The resonance previously assigned as the para-hydrogen triplet is an overlap of two C6H4 doublets.

IR (thin film on KBr plate): νCO = 1996 cm-1; νNH = 3225 cm-1. 1H NMR (CD3CN, δ, 25 °C): 10.18 and 10.14 (each 1H, each a br s, NH of isomers), 8.00, 7.98, 7.91, 6.50 (each 2H, each a d, 3JHH = 2 Hz, Tp CH 3 and 5 position), 7.52–6.98 (38H total, overlap of resonances for Tp CH 3 and 5 position, PPh3 and C6H4), 6.90 (2H, overlapping d's, C6H4), 6.30, 6.10, 6.02 (each 2H, each a t, 3JHH = 2 Hz, Tp CH 4 position), NOTE: At room temperature two upfield resonances (~5 ppm) due to the C6H4 rings are broadened into the baseline due to a fluxional process. 13C{1H} NMR (CD3CN, δ, 25 °C): 203.0 (d, 2JPC = 15 Hz, CO), 171.6 (s, ipso carbon of C6H4), 146.9, 145.8, 144.9, 139.7, 138.3, 137.9 (each a s, Tp CH's), 137.2, 134.9, 134.8, 132.6, 130.3, 130.2, 129.8, 129.5, 128.5, 126.6 (PPh3 and C6H4 resonances), 108.5, 108.3, 107.9 (each a s, Tp 4 position CH's). 31P{1H} NMR (CD3CN, δ, 25 °C): 42.2.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.