Additions and corrections
Enantioselective desymmetrization of prochiral cyclohexanone derivatives via the organocatalytic direct aldol reaction
Jun Jiang, Long He, Shi-Wei Luo, Lin-Feng Cun and Liu-Zhu Gong
Chem. Commun., 2007, 736–738 (DOI: 10.1039/b615043h) Amendment published 19th September 2008
The authors being aware of the proline-catalyzed aldol reaction of two six-membered meso cyclic ketones, the statement "Surprisingly, there has been, to date, no report of using organocatalytic asymmetric aldol reactions to break the symmetry of prochiral and meso ketones 8p" should be revised to "Surprisingly, there has been, to date, no systematic study on using organocatalytic asymmetric aldol reactions to break the symmetry of prochiral and meso ketones with the exception of the proline-catalyzed aldol reaction of two six-membered meso cyclic ketones 8p,q". Accordingly, an additional reference (M. Majewski, I. Niewczas and N. Palyam, Synlett, 2006, 2387) should be included as ref. 8q.
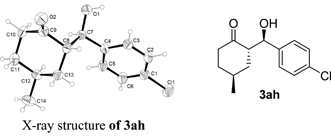
According to the X-ray structure of 3ah that has been obtained,† the authors found that the stereochemistry of the desymmetrization products was assigned incorrectly by comparing the optical rotation of the product 4-tert-butyl-2-(hydroxy(phenyl)methyl)cyclohexanone reported previously (ref. 17, J. Busch-Petersen and E. J. Corey, Tetrahedron Lett., 2000, 41, 6941). The stereochemistry of 3ah should be corrected as shown below:

Accordingly, the structures of 3aa in Table 1, 3aa-al in Table 2 and 3aa-ea in Table 3 have been reassigned by analogy as shown below:
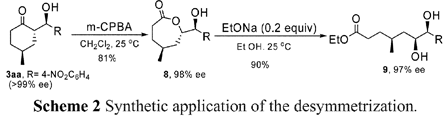
Scheme 2 should be corrected as shown below:
The authors apologise for these errors and thank those readers who pointed them out.
† CCDC reference number 697513.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article