Additions and corrections
Reversible modulation of -association between 3,6-disubstituted carbazole ligands in a multistep assembling process
Norie Inukai, Junpei Yuasa and Tsuyoshi Kawai
Chem. Commun., 2010, 3929–3931 (DOI: 10.1039/c001287d) Amendment published 27 July 2012
Background
We have previously reported that carbazole ligand (Im2Cz) formed a dimer complex having multiple pi-stacking interactions in Chem. Commun (DOI: 10.1039/c001287d). During the course of our work, we performed NMR titration of Im2Cz by zinc ion (Zn2+) to investigate complex formation between Im2Cz and Zn2+. The NMR titration analysis revealed that the doublet peak due to aromatic proton of the carbazole ring is upfield shifted considerably from 7.75 to 5.73 ppm (Fig. 2a) in the presence of Zn2+, suggesting formation of pi-stacking interactions between carbazole ligands during complexation with Zn2+. The NMR titration analysis also suggested two sets of carbazole rings and four sets of imidazole rings of the Zn2+ complex located in different chemical environments. According to the detailed NMR analysis, we initially assumed two types of dimer complex, [(Im2Cz)4-Zn2+]2 and [(Im2Cz)2-Zn2+]2, were possible structures for the Zn2+ complex that have pi-stacking interactions. In order to determine the stoichiometry of the Zn2+ complex, we performed UV-Vis. titration of Im2Cz by Zn2+, where the absorbance change at lambda = 365 nm is plotted against [Zn2+]/[Im2Cz]0 (Fig. 1). The titration analysis suggests the stoichiometry of four Im2Cz ligands per Zn2+ at [Zn2+]/[Im2Cz]0 = 0.25 and three Im2Cz ligands per Zn2+ at [Zn2+]/[Im2Cz]0 = 0.33. This result suggests formation of [(Im2Cz)4-Zn2+]2, which converts to (Im2Cz)3-Zn2+ at [Zn2+]/[Im2Cz]0 = 0.33. Thus, we have finally concluded [(Im2Cz)4-Zn2+]2 to be the most likely substance of the dimer complex.
Correction and Additional Information
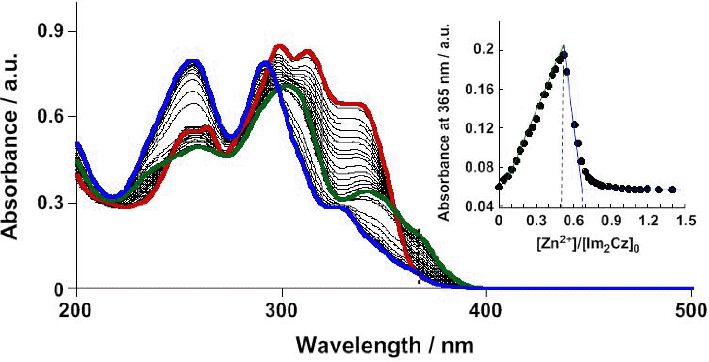
We recently become aware of the past evaluation of the concentration of Im2Cz (=[Im2Cz]0) being erroneous. Then, we re-performed the titration twice and observed good reproducibility with the corrected concentration. The corrected results of the UV-vis titration are provided in Fig. C1. This corrected-result suggests the stoichiometry of two Im2Cz ligands per Zn2+ at [Zn2+]/[Im2Cz]0 = 0.50 and three Im2Cz ligands per two Zn2+ ions at [Zn2+]/[Im2Cz]0 = 0.66, being consistent with formation of [(Im2Cz)2-Zn2+]2 and [(Im2Cz)3-(Zn2+)2], respectively.
Following data are also provided for supporting the conclusions.
Im2Cz, 1-ethyl-4,7-bis(2-(1-methylimidazol-2-yl)-ethynyl)-carbazole ( =56.7x103 M cm-1), 1H NMR (300 MHz, CD3CN): 8.31 (s, 2H), 7.64 (d, J = 8.4 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H), 7.06 (s, 2H), 7.00 (s, 2H), 4.35 (q, J = 7.1 Hz, 2H), 3.76 (s, 6H), 1.33 (t, J = 7.1 Hz, 3H). Anal. Calcd. for C26H21N5: C, 77.40; H, 5.25; N, 17.36. Found: C, 77.31; H, 4.98; N, 17.51. 13C NMR (100 MHz, CD3CN): 141.42, 133.28, 130.62, 129.95, 125.35, 123.25, 123.02, 118.26, 113.23, 110.72, 94.00, 78.33, 38.71, 34.01, 14.05 ppm. HRMS (ESI-MS) m/z Calcd. for C26H21N5 [M]+: 404.1875, found 404.1874. ESI-MS signals m/z = 1019.23 (m/z = 1019.24 calcd. for {[Zn(Im2Cz)2](OSO2CF3)}+) and m/z = 1784.25 (m/z =1784.25 calcd. for {[Zn2 (Im2Cz)3](OSO2CF3)3}+) were observed in acetone solutions, [Im2Cz]total = 2.1 x 10-3 mol dm-3 with [Zn2+]total = 1.2 x 10-3 mol dm-3 and 2.8 × 10-3 mol dm-3, respectively.

Fig. C1 Observed UV-vis absorption spectral changes upon addition of Zn2+ [0 mol dm-3 (red line) - 7.8 x 10-5 mol dm-3 (green line) - 1.6 x 10-4 mol dm-3 (blue line)] to a MeCN solution of Im2Cz (1.5 x 10-4 mol dm-3) at 298 K (1 mm path length). Inset: Plot of absorbance at lambda = 365 nm vs. [Zn2+]/[Im2Cz]0, where [Im2Cz]0 is the initial concentration of Im2Cz (1.5 x 10-4 mol dm-3).
The Royal Society of Chemistry apologises for this error and any consequent inconvenience to authors and readers.
Back to article