Additions and corrections
Bulk crystal growth and characterization of imidazolium L-tartrate (IMLT): a novel organic nonlinear optical material with a high laser-induced damage threshold
Chengmin Ji, Tianliang Chen, Zhihua Sun, Yan Ge, Wenxiong Lin, Junhua Luo,* Qian Shi* and Maochun Hong
CrystEngComm, 2013, 15, 2157–2162 (DOI: 10.1039/C3CE26942F). Amendment published 30th April 2013.
The authors would like to correct references in the original manuscript. In addition, a corrected crystal structural description for the title compound IMLT is provided.
1. The
author details of reference 15 were incorrect in the original manuscript and
should be amended as follows:
C. B. Aakeroy, P. B. Hitchcock, J. Mater.
Chem., 1993, 3, 1129.
2. The reference “C. B. Aakeroy, P. B. Hitchcock. K. R. Seddon, J. Chem. Soc., Chem. Commun., 1992, 553–555” should be cited as reference 5 in the sentence of “In the past two decades, many researchers have taken L-tartaric acid as a small organic molecules with large dipole moment, weak van der Waals, hydrogen bonds, wide transparency ranges and chiral structure to assemble the acentric crystal architectures.4,5” in the original manuscript.
3. The correction of the crystal structure description for the title compound IMLT is listed as follows and Fig. 3c is added (page 2159):
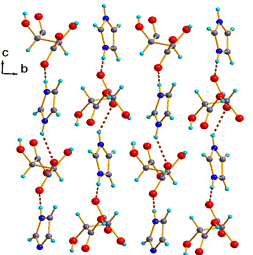
Fig. 3(c). Crystal structure packing viewed along the a-axis.
The text, “The L-tartrate anions display a zig-zag configuration with a C–C–C–C torsion angle of almost 180° to form infinite chains via O–H…O hydrogen bonds between the anions through a head-to-tail arrangement. These chains are then cross-linked in the a–b plane related by the 21 screw axis parallel to b, via O–H…O hydrogen bonds, constructing an infinite 2D sheet as shown in Fig. 3(b). In addition, the imidazolium cations provide a cross-link between the anionic “scaffolding” since each cation is engaged in hydrogen bonds between the adjacent anionic layers parallel to the c-axis, shown in Fig. 3(a), resulting in an interlinked anion–cation layer in the a–c plane with a hydrogen-bonded supramolecular network.”
in the left column of page 2159 is replaced by:
“The L-tartrate anions are bound to each other through the O–H•••O hydrogen bonds between the carboxyl groups and present 1D infinite zigzag arrangements along a-axis. On the other hand, in the b–c plane the imidazolium cations and the L-tartrate anions are alternately connected through by the N(2)–H•••O(1) and O(2)–H•••N(1) hydrogen bonds to form anti-parallel links with the hydrogen bond chains correlated to the 21 screw axis parallel to b-axis as shown in Fig. 3(c). In addition, the 2D network anion–cation layer constructed through the O–H•••O and N–H•••O hydrogen bonds between the anions and cations in the a–c plane are connected reversely along b-axis in the a–b plane via O–H•••O hydrogen bonds resulting in a 3D supramolecular network (Fig. 3(a) and 3(b)).”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article