Additions and corrections
The S0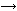 S1
cavity ring-down absorption spectrum of jet-cooled azulene: dependence
of internal conversion on the excess energy
S1
cavity ring-down absorption spectrum of jet-cooled azulene: dependence
of internal conversion on the excess energy
| Phys. Chem. Chem. Phys., 1999, 5121 | |
Additions and corrections The S0 |
A. A. Ruth, E.-K. Kim and A. Hese
Phys. Chem. Chem. Phys., 1, 5121. (DOI: 10.1039/a906344g) . Amendment published on the Web 21st July 2000
The authors wish to apologise for an unfortunate but severe mistake in
the above publication in the evaluation of the homogeneous linewidths.
Instead of the full width at half maximum the half width
at half maximum was used for the Lorentzian lineshape in the published
evaluation procedure. The correct homogeneous linewidth for the S0,0 S1,0
transition of jet-cooled azulene reported by us to be 2.0 + 0.25 cm–1
(in the text and also in Fig. 3a) should therefore be 4.0 + 0.5 cm–1
corresponding to an S1,0 lifetime of
S1,0
transition of jet-cooled azulene reported by us to be 2.0 + 0.25 cm–1
(in the text and also in Fig. 3a) should therefore be 4.0 + 0.5 cm–1
corresponding to an S1,0 lifetime of  (S1)=
1.30 ps with error limits of +0.22 and –0.12 ps. Consequently all
values for
(S1)=
1.30 ps with error limits of +0.22 and –0.12 ps. Consequently all
values for 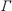 hom
of vibronic transitions S0,0
hom
of vibronic transitions S0,0 S1,v
in the fifth column of Table 1 should also be larger by a factor of 2.
This mistake affects the two sections on the comparison with published S1
lifetimes in the discussion: Our previous conclusion that the molecular
environment does not significantly perturb the azulene dynamics must be
revised; although our measurement yields a slightly larger value for
S1,v
in the fifth column of Table 1 should also be larger by a factor of 2.
This mistake affects the two sections on the comparison with published S1
lifetimes in the discussion: Our previous conclusion that the molecular
environment does not significantly perturb the azulene dynamics must be
revised; although our measurement yields a slightly larger value for  (S1.0)
of jet-cooled azulene than previously reported S1,0
lifetimes (0.8 + 0.2,1
1.1 + 0.2,2 1.1
3 ps), this
result corroborates1
a significant medium effect on the intramolecular relaxation of the S1
electronic origin when compared to measurements in solid matrix [
(S1.0)
of jet-cooled azulene than previously reported S1,0
lifetimes (0.8 + 0.2,1
1.1 + 0.2,2 1.1
3 ps), this
result corroborates1
a significant medium effect on the intramolecular relaxation of the S1
electronic origin when compared to measurements in solid matrix [ (S1,0)~
2.6 or 3.3 ps]4,5
at low temperatures or with time domain measurements in solution (1.0,6
1.7 ps 7).
Shorter decay times in the isolated molecule still exist at an excess
energy of
(S1,0)~
2.6 or 3.3 ps]4,5
at low temperatures or with time domain measurements in solution (1.0,6
1.7 ps 7).
Shorter decay times in the isolated molecule still exist at an excess
energy of  2000 cm–1
where our value for the IC decay time is now ~0.5 ps in comparison to time
domain measurements yielding 0.9 ps in the jet.8
2000 cm–1
where our value for the IC decay time is now ~0.5 ps in comparison to time
domain measurements yielding 0.9 ps in the jet.8
Finally, statements in the Introduction should be read with caution and the corrected value for the S1,0 lifetime in mind!
We would like to genuinely apologise for this error and would like to thank Dr C. Cossart-Magos and Prof. J. Jortner sincerely for bringing this flaw to our attention.
| 1 | A. Amirav and J. Jortner, J. Chem. Phys., 1984, 81, 4200. | |
| 2 | T. Suzuki and M. Ito, J. Phys. Chem., 1987, 91, 3537. | |
| 3 | S. K. Kulkarmi and J. E. Kenny, J. Chem. Phys., 1988, 89, 4441. | |
| 4 | R. M. Hochstrasser and T.-Y. Li, J. Mol. Spectrosc., 1972, 41, 297. | |
| 5 | R. M. Hochstrasser and C. A. Nyi, J. Chem. Phys., 1979, 70, 1112. | |
| 6 | B. D. Wagner, M. Szymanski and R. P. Steer, J. Chem. Phys., 1993, 98, 301. | |
| 7 | A. J. Wurzer, T. Wilhelm, J. Piel and E. Riedle, Chem. Phys. Lett., 1999, 299, 296. | |
| 8 | E. W.-G. Diau, S. DeFeyter and A. H. Zewail, J. Chem. Phys., 1999, 110, 9785. |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.