Additions and corrections
QCT and QM calculations of the Cl(2P) + NH3 reaction: influence of the reactant well on the dynamics
M. Monge-Palacios, M. Yang and J. Espinosa-García
Phys. Chem. Chem. Phys., 2012, 14, 4824–4834 (DOI: 10.1039/C2CP00008C). Amendment published 18th October 2012.
Our purpose here is to add some needed clarifying remarks and make some corrections to the previously published paper, which call into question the conclusions we drew about the mechanism of the reaction at low energies.
In that original manuscript, we performed quasi-classical trajectory (QCT) and reduced-dimensional quantum mechanical (7D-QM) calculations that were aimed at understanding the reactivity and mechanism of the title reaction, which evolves through deep wells in the entry and exit channels. The QCT calculations showed strong enhancement of the reaction probability below the reaction threshold. This was due to repeated encounters between the reactants in the entry well, and, as a consequence, the vibrational energy in the NH2 product appears below its zero-point energy, which is quantum mechanically forbidden. Thus, in these calculations, a part of the leaked vibrational energy is used to artificially promote the reaction. However, our 7D-QM calculations also reproduced this behaviour at low energies, due now to possible resonances in the entry well, and this appeared to lend confidence to the QCT results, although it was already clear in the original paper that the latter overestimated the influence at low energies due to including unphysical vibrational→translational energy transfer. But an oversight on the part of the authors resulted in a mistake in the 7D-QM calculations, so that it was clear that the peak in the reaction probability at low energies might have been wrong in the reduced-dimensional quantum dynamics. In particular, the parameters defining the dividing surface were wrongly selected, with the result that some wavepackets were included in the flux analysis even though they do not pass through the transition state.
We have therefore repeated the reduced-dimensional QM calculations by setting the dividing surface at r = 3.5 Bohr. Because of the double well in this reaction, we enlarged the expansion for the coordinate r, using an extremely large basis set in the new calculation. In the L-shape technique used for the coordinates R and r, a total of 480 basis sine functions ranging from 2.5 to 23.0 Bohr were used for the R basis set expansion, with 410 nodes in the interaction region. The range of r was set to be from 1.0 Bohr to 21.0 Bohr to include the product well, and 5 and 140 basis functions were used in the asymptotic and interaction regions, respectively. For the NH2 bending mode, 1 PODVR node was used. The size of the rotational basis function set is controlled by the parameters Jmax = 48, lmax = 30, jmax = 18, and kmax = 18. With the consideration of parity and C2v symmetry, the size of that set was 54 979, and the number of nodes for its integration was 654 493. The size of the total basis function set was 3.175 × 109, and the number of nodes 3.78 × 1010. Due to the deep well in the entry channel and the heavy mass of the Cl atom, the entire propagation time was that corresponding to 25 000 a.u. with a step size of 10 a.u. to provide total reaction probabilities as a function of the translational energy for Jtot = 0.
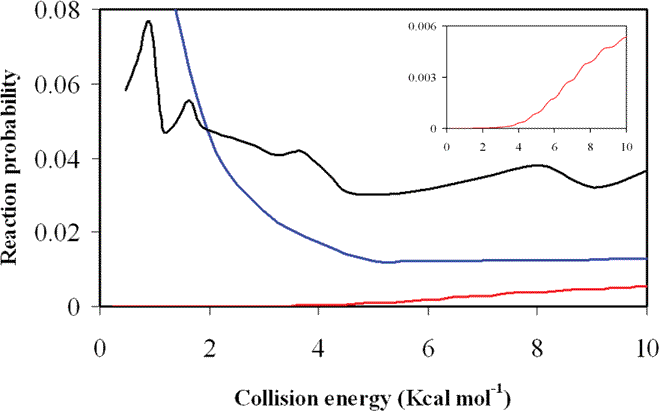
A very different picture is obtained from the new QM calculation. Figure 1 shows the reaction probability as a function of the collision energy for the 7D-QM calculations (J = 0). In addition the QCT (b = 0) and the old 7D-QM (J = 0) results are also included for comparison. Using the new QM results we find that the reaction probability rises near the reaction threshold, and then increase smoothly with collision energy. In particular, this means that there is no resonance at low energies, and that the entry well does not support any bound state.
In sum, the new QM calculations show the behaviour typical of reactions with a barrier, and neither resonances nor any other quantum effects are observed below the reaction barrier. Hence the strong peak observed at low energies with the QCT method is an artefact due to the unphysical vibrational→translational energy transfer produced in the entry well.

Figure 1. Reaction probability as a function of the collision energy (kcal mol¬1) using the PES-2010 surface for the Cl + NH3 reaction. Red curve: new 7D-QM calculations (J = 0); blue curve: QCT calculations (b = 0); and black curve: old 7D-QM calculations (J = 0). For a clearer comparison, we also include the new 7D-QM results as an insert.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article