1H NMR (CD2Cl2 298 K selected)
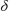 7.72–6.95 (m, ArH), -26.25 (br s, IrH). 31P{1H} NMR (CD2Cl2 298 K)
7.72–6.95 (m, ArH), -26.25 (br s, IrH). 31P{1H} NMR (CD2Cl2 298 K) 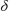 23.1 (br s).
23.1 (br s).Additions and corrections
The role of halogenated carborane monoanions in olefin hydrogenation catalysed by cationic iridium phosphine complexes
Gemma L. Moxham, Thomas M. Douglas, Simon K. Brayshaw, Gabriele Kociok-Köhn, John P. Lowe and Andrew S. Weller*
Dalton Trans., 2006 (DOI: 10.1039/b612049k). Amendment published 15th February 2007.
The NMR data for the complexes [Ir(PPh3)2H2(Ln)][Y] (Y = [1-closo-CB11H6Cl6]- 1c/c', [BArF4]- 4c/c', L = CD2Cl2 or agostic interactions) were reported incorrectly. Due to trace adventitious water in the sample the NMR data reported were actually for an approximate 50 : 50 mixture of the aqua complexes [Ir(PPh3)2H2(L)(H2O)][Y] and [Ir(PPh3)2H2(Ln)][Y]. Identification of the aqua complexes comes from addition of small amounts of H2O to the samples. The new data still do not allow the unambiguous determination of the identity of L (either CD2Cl2 or an agostic interaction), and our overall conclusions remain the same in as much as the anion in these systems does not bind to the metal centre. The Authors apologise for this oversight in the original publication.
[Ir(PPh3)2H2(Ln)][BArF4] (4c) and [Ir(PPh3)2H2(L)(H2O)][BArF4] (~50/50 mixture)
1H NMR (CD2Cl2 298 K selected) 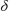 7.72–6.95 (m, ArH), -26.25 (br s, IrH). 31P{1H} NMR (CD2Cl2 298 K)
7.72–6.95 (m, ArH), -26.25 (br s, IrH). 31P{1H} NMR (CD2Cl2 298 K) 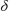 23.1 (br s).
23.1 (br s).
[Ir(PPh3)2H2(Ln)][BArF4] (4c)
1H NMR (CD2Cl2 250 K selected) 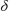 -25.57 (t, J(PH) 15 Hz, IrH). 31P{1H} NMR (CD2Cl2 250 K selected)
-25.57 (t, J(PH) 15 Hz, IrH). 31P{1H} NMR (CD2Cl2 250 K selected) 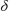 23.1 (s). 1H NMR (CD2Cl2 200 K selected)
23.1 (s). 1H NMR (CD2Cl2 200 K selected) 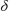 -25.16 (t, J(PH) 15 Hz, IrH). 31P{1H} NMR (CD2Cl2 200 K selected)
-25.16 (t, J(PH) 15 Hz, IrH). 31P{1H} NMR (CD2Cl2 200 K selected) 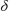 23.2 (s).
23.2 (s).
[Ir(PPh3)2H2(L)(H2O)][BArF4]
1H NMR (CD2Cl2 250 K selected) 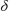 -27.81 (br s, IrH). 31P{1H} NMR (CD2Cl2 250 K selected)
-27.81 (br s, IrH). 31P{1H} NMR (CD2Cl2 250 K selected) 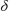 27.0 (s). 1H NMR (CD2Cl2 200 K selected)
27.0 (s). 1H NMR (CD2Cl2 200 K selected) 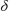 -26.76 (br s, IrH), -28.18 (br s, IrH). 31P{1H} NMR (CD2Cl2 200 K selected)
-26.76 (br s, IrH), -28.18 (br s, IrH). 31P{1H} NMR (CD2Cl2 200 K selected) 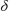 27.0 (s).
27.0 (s).
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.