Additions and corrections
Sn3 and Sn10 sulfonate–oxide–hydroxide clusters with two different sulfonate
binding modes
Ganesan Prabusankar, Bernard Jousseaume, Thierry Toupance and Hassan Allouchi
Dalton Trans., 2007 (DOI: 10.1039/b706377f). Amendment published 21st May 2008.
In Scheme 1, TSA should be changed to 2,5-Me2C6H3SO3H on the lower arrow.
The following paragraph replaces the third paragraph of page 3122:
As the cleavage of tin–aryl groups resulting in the presence of inorganic tins in 2 could originate from the prolonged treatment of 1 under acidic conditions, we undertook a study of the reaction of 1 with bulkier 2,5-dimethylbenzenesulfonic acid under milder conditions: 1 was first subjected to hydrolysis and then reacted with 2,5-dimethylbenzenesulfonic acid. So, treatment of 1 with twelve equivalents of water in THF resulted in the cleavage of the tin–alkynyl bonds. Then an equimolar reaction of 2,5-dimethylbenzenesulfonic acid at 72 °C for an additional 6 h yielded the colourless crystals of 3†¶ {[2,4,6-iPr3C6H2Sn-(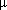 2-OH)-(
2-OH)-(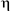 2
2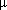 2)-2,5-Me2C6H3SO3]3(
2)-2,5-Me2C6H3SO3]3(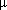 3-O)}+ 2,5-Me2C6H3SO3-, 2 toluene. The IR spectrum of 3 was devoid of any acetylenic absorption band and the strong bands at 3476 and 3416 cm-1 indicated the presence of associated OH groups in the molecule. Compound 3 is unstable in solution and undergoes slow decomposition to yield a soluble NMR silent tin-oxo polymer.16 The CPMAS 119Sn investigation of 3 indicated the existence of only one tin site in the molecule with a shift of
3-O)}+ 2,5-Me2C6H3SO3-, 2 toluene. The IR spectrum of 3 was devoid of any acetylenic absorption band and the strong bands at 3476 and 3416 cm-1 indicated the presence of associated OH groups in the molecule. Compound 3 is unstable in solution and undergoes slow decomposition to yield a soluble NMR silent tin-oxo polymer.16 The CPMAS 119Sn investigation of 3 indicated the existence of only one tin site in the molecule with a shift of 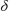 = -600 ppm, characteristic of a hexacoordinated monoorganotin. So, milder reaction conditions resulted in the total preservation of organic groups linked to the metal. Compound 3 consists of a six membered {[2,4,6-iPr3C6H2Sn-(
= -600 ppm, characteristic of a hexacoordinated monoorganotin. So, milder reaction conditions resulted in the total preservation of organic groups linked to the metal. Compound 3 consists of a six membered {[2,4,6-iPr3C6H2Sn-(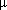 2-OH)]3(
2-OH)]3(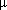 3-O)}+ cation with three bridging
3-O)}+ cation with three bridging 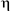 2
2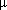 2 sulfonate fragments and a central
2 sulfonate fragments and a central 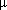 3 oxygen atom (Fig. 2). The tin atoms are hexa-coordinated. The counteranion consists of a 2,5-dimethylbenzenesulfonate anion coordinated to the hydrogen atoms of the hydroxyl groups. Two kinds of sulfonate binding are present in 3, as in 2, but different from those of 2: an electrostatic one involving three hydroxyl groups and a bridging covalent one involving two tin atoms. The coexistence of both these modes in the same cluster is, to the best of our knowledge, unprecedented.
3 oxygen atom (Fig. 2). The tin atoms are hexa-coordinated. The counteranion consists of a 2,5-dimethylbenzenesulfonate anion coordinated to the hydrogen atoms of the hydroxyl groups. Two kinds of sulfonate binding are present in 3, as in 2, but different from those of 2: an electrostatic one involving three hydroxyl groups and a bridging covalent one involving two tin atoms. The coexistence of both these modes in the same cluster is, to the best of our knowledge, unprecedented.
The following paragraph replaces the second paragraph of page 3123:
3: H2O (0.9 g, 49.2 mmol) was added dropwise to a stirred solution of 1 (1.8 g, 4 mmol) in a mixture THF–toluene (3 : 1) (30 mL) at RT. The reaction mixture was left at RT for 9 h. 2,5-Dimethylbenzenesulfonic acid dihydrate (0.8 g, 4 mmol) was added and the solution was heated under reflux for 6 h. The solvents were removed under reduced pressure to result in a white solid. It was dissolved in toluene and the resulting solution filtered to give the colorless crystals of 3 which decompose slowly on standing. (1.2 g, 47%); mp 155-157 °C (decomp.); 1H NMR (CDCl3, 250 MHz): 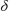 1.08–1.28 (dd, 54H, iPr-CH3), 2.13–2.43 (m, 24H, Ar-CH3), 2.69–3.44 (m, 9H iPr-CH), 3.26 (s, OH), 6.82–7.16, 7.48, 7.50, 7.84 (m, aryl-CH), 8.95, 9.66 (s, OH) ppm; CP MAS 119Sn NMR:
1.08–1.28 (dd, 54H, iPr-CH3), 2.13–2.43 (m, 24H, Ar-CH3), 2.69–3.44 (m, 9H iPr-CH), 3.26 (s, OH), 6.82–7.16, 7.48, 7.50, 7.84 (m, aryl-CH), 8.95, 9.66 (s, OH) ppm; CP MAS 119Sn NMR: 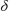 = -600 ppm; IR(KBr):
= -600 ppm; IR(KBr):  = 3476s, 3416s, 2963vs, 2913m, 2872m, 1596w, 1560w, 1492w, 1464m, 1420w, 1385m, 1364m, 1288s, 1270s,1279s, 1148m, 1077vs, 1015s, 995s, 881m, 813w, 733w, 703m, 621vs cm-1. Crystallographic data: 3a: C87.5H119.5O16S4Sn3, M = 1911.64, monoclinic, space group P-1 (no. 2), a =14.5396(1) Å, b =17.6961(1) Å, c = 18.8745(1) Å,
= 3476s, 3416s, 2963vs, 2913m, 2872m, 1596w, 1560w, 1492w, 1464m, 1420w, 1385m, 1364m, 1288s, 1270s,1279s, 1148m, 1077vs, 1015s, 995s, 881m, 813w, 733w, 703m, 621vs cm-1. Crystallographic data: 3a: C87.5H119.5O16S4Sn3, M = 1911.64, monoclinic, space group P-1 (no. 2), a =14.5396(1) Å, b =17.6961(1) Å, c = 18.8745(1) Å, 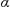 = 94.1887(3)°,
= 94.1887(3)°, 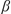 = 99.7233(3)°,
= 99.7233(3)°, 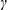 = 109.3132(3)°, V = 4473.59(5) Å3, Z = 2,
= 109.3132(3)°, V = 4473.59(5) Å3, Z = 2, 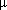 calcd =1.419 g cm-3, T = 150(2) K, F(000) = 1973,
calcd =1.419 g cm-3, T = 150(2) K, F(000) = 1973, 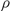 (Mo-K
(Mo-K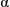 ) = 0.986 mm-1, block crystal, 40 387 reflections measured, 20 465 unique (Rint =0.0126), 1000 parameters, R1 =0.0329 (I > 2
) = 0.986 mm-1, block crystal, 40 387 reflections measured, 20 465 unique (Rint =0.0126), 1000 parameters, R1 =0.0329 (I > 2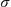 (I)), wR2 = 0.0829 (I > 2
(I)), wR2 = 0.0829 (I > 2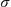 (I)), GOF = 1.033.
(I)), GOF = 1.033.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back
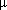 2-OH)-(
2-OH)-(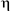 2
2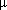 2)-2,5-Me2C6H3SO3]3(
2)-2,5-Me2C6H3SO3]3(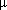 3-O)}+ 2,5-Me2C6H3SO3-, 2 toluene. The IR spectrum of 3 was devoid of any acetylenic absorption band and the strong bands at 3476 and 3416 cm-1 indicated the presence of associated OH groups in the molecule. Compound 3 is unstable in solution and undergoes slow decomposition to yield a soluble NMR silent tin-oxo polymer.16 The CPMAS 119Sn investigation of 3 indicated the existence of only one tin site in the molecule with a shift of
3-O)}+ 2,5-Me2C6H3SO3-, 2 toluene. The IR spectrum of 3 was devoid of any acetylenic absorption band and the strong bands at 3476 and 3416 cm-1 indicated the presence of associated OH groups in the molecule. Compound 3 is unstable in solution and undergoes slow decomposition to yield a soluble NMR silent tin-oxo polymer.16 The CPMAS 119Sn investigation of 3 indicated the existence of only one tin site in the molecule with a shift of 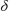 = -600 ppm, characteristic of a hexacoordinated monoorganotin. So, milder reaction conditions resulted in the total preservation of organic groups linked to the metal. Compound 3 consists of a six membered {[2,4,6-iPr3C6H2Sn-(
= -600 ppm, characteristic of a hexacoordinated monoorganotin. So, milder reaction conditions resulted in the total preservation of organic groups linked to the metal. Compound 3 consists of a six membered {[2,4,6-iPr3C6H2Sn-(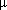 2-OH)]3(
2-OH)]3(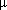 3-O)}+ cation with three bridging
3-O)}+ cation with three bridging 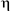 2
2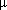 2 sulfonate fragments and a central
2 sulfonate fragments and a central 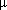 3 oxygen atom (Fig. 2). The tin atoms are hexa-coordinated. The counteranion consists of a 2,5-dimethylbenzenesulfonate anion coordinated to the hydrogen atoms of the hydroxyl groups. Two kinds of sulfonate binding are present in 3, as in 2, but different from those of 2: an electrostatic one involving three hydroxyl groups and a bridging covalent one involving two tin atoms. The coexistence of both these modes in the same cluster is, to the best of our knowledge, unprecedented.
3 oxygen atom (Fig. 2). The tin atoms are hexa-coordinated. The counteranion consists of a 2,5-dimethylbenzenesulfonate anion coordinated to the hydrogen atoms of the hydroxyl groups. Two kinds of sulfonate binding are present in 3, as in 2, but different from those of 2: an electrostatic one involving three hydroxyl groups and a bridging covalent one involving two tin atoms. The coexistence of both these modes in the same cluster is, to the best of our knowledge, unprecedented. = 3476s, 3416s, 2963vs, 2913m, 2872m, 1596w, 1560w, 1492w, 1464m, 1420w, 1385m, 1364m, 1288s, 1270s,1279s, 1148m, 1077vs, 1015s, 995s, 881m, 813w, 733w, 703m, 621vs cm-1. Crystallographic data: 3a: C87.5H119.5O16S4Sn3, M = 1911.64, monoclinic, space group P-1 (no. 2), a =14.5396(1) Å, b =17.6961(1) Å, c = 18.8745(1) Å,
= 3476s, 3416s, 2963vs, 2913m, 2872m, 1596w, 1560w, 1492w, 1464m, 1420w, 1385m, 1364m, 1288s, 1270s,1279s, 1148m, 1077vs, 1015s, 995s, 881m, 813w, 733w, 703m, 621vs cm-1. Crystallographic data: 3a: C87.5H119.5O16S4Sn3, M = 1911.64, monoclinic, space group P-1 (no. 2), a =14.5396(1) Å, b =17.6961(1) Å, c = 18.8745(1) Å, 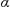 = 94.1887(3)°,
= 94.1887(3)°, 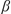 = 99.7233(3)°,
= 99.7233(3)°, 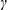 = 109.3132(3)°, V = 4473.59(5) Å3, Z = 2,
= 109.3132(3)°, V = 4473.59(5) Å3, Z = 2, 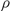 (Mo-K
(Mo-K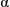 ) = 0.986 mm-1, block crystal, 40 387 reflections measured, 20 465 unique (Rint =0.0126), 1000 parameters, R1 =0.0329 (I > 2
) = 0.986 mm-1, block crystal, 40 387 reflections measured, 20 465 unique (Rint =0.0126), 1000 parameters, R1 =0.0329 (I > 2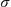 (I)), wR2 = 0.0829 (I > 2
(I)), wR2 = 0.0829 (I > 2