Additions and corrections
Negishi cross-coupling reaction catalyzed by an aliphatic, phosphine based pincer complex of palladium. biaryl formation via cationic pincer-type PdIV intermediates
Roman Gerber, Olivier Blacque and Christian M. Frech
Dalton Trans., 2011, 40, 8996 (DOI: 10.1039/C1DT10398A). Amendment published 26th October 2011.
The authors apologise that in the above article all of the conversions and yields for the Negishi cross-coupling reactions are the same in Tables 1-4. The corrected tables with correct conversions and yields are displayed below.
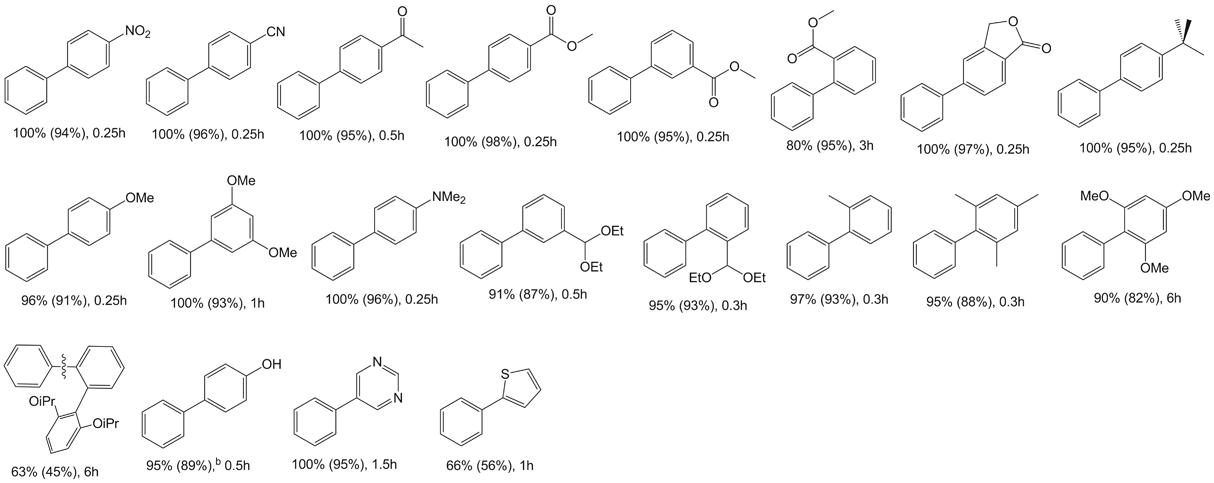
Table 1 Negishi cross-coupling reactions of aryl bromides with diphenylzinc catalyzed by 1a

aReaction conditions: 2.0 mmol aryl halide, 1.4 mmol diphenylzinc, 4 ml NMP, catalyst (0.01 mol%) added in solution (dioxane), reaction performed at 100°C. Conversions determined by GC/MS, based on aryl halide. b2.8 mmol of diphenylzinc was used.
Table 2 Negishi cross-coupling reactions of aryl bromides with bis(4-methoxyphenyl)zinc and bis[4-(dimethylamino)phenyl]zinc catalyzed by 1a
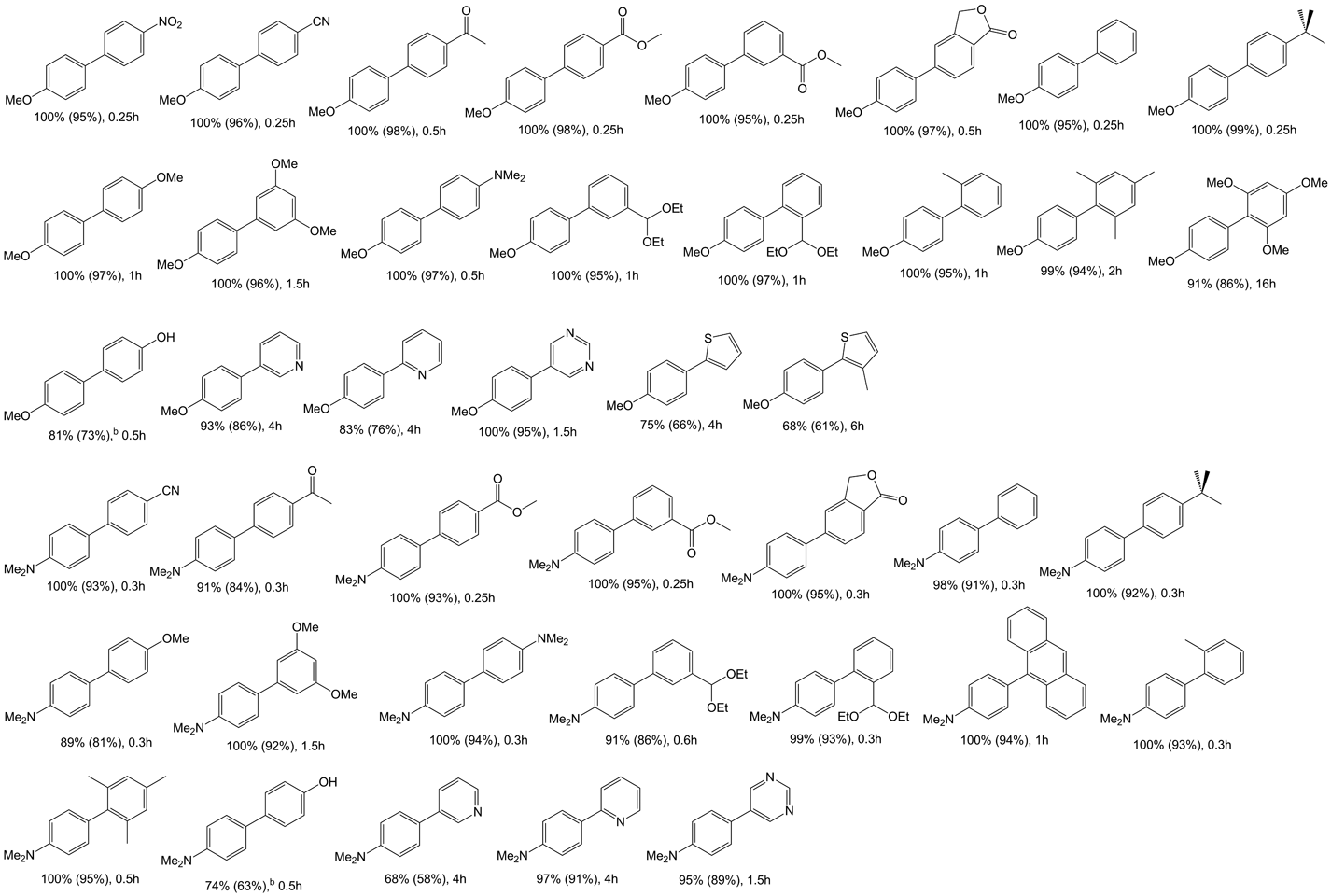
aReaction conditions: 2.0 mmol aryl halide, 1.4 mmol diphenylzinc, 4 ml NMP, catalyst (0.01 mol%) added in solution (dioxane), reaction performed at 100°C. Conversions determined by GC/MS, based on aryl halide. b2.8 mmol of the diarylzinc was used.
Table 3 Negishi cross-coupling reactions of aryl bromides with bis(2-methylphenyl)zinc and bis(2,4,6-trimethylphenyl)zinc catalyzed by 1a
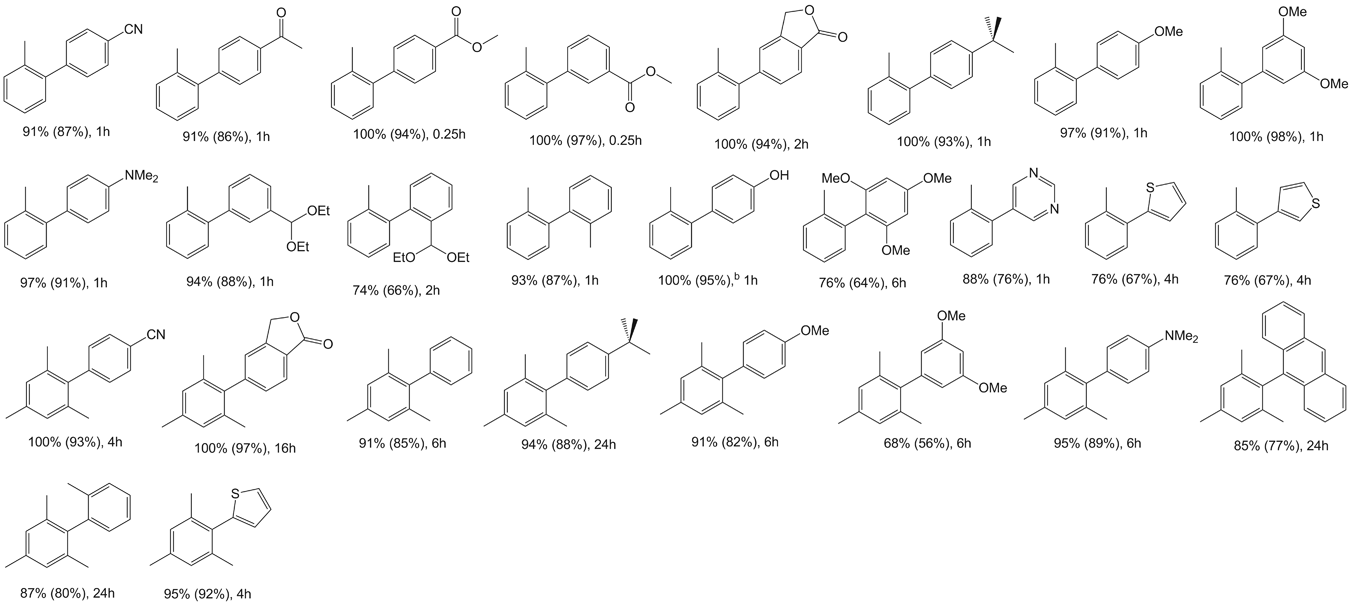
aReaction conditions: 2.0 mmol aryl halide, 1.4 mmol diphenylzinc, 4 ml NMP, catalyst (0.01 mol%) added in solution (dioxane), reaction performed at 100°C. Conversions determined by GC/MS, based on aryl halide. b2.8 mmol of the diarylzinc was used.
Table 4 Negishi cross-coupling reactions of aryl bromides with dithiophen-3-ylzinc catalyzed by 1a
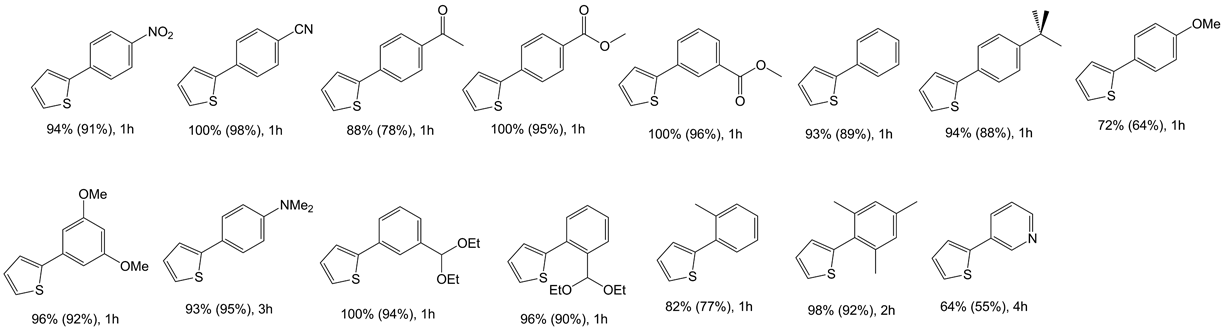
aReaction conditions: 2.0 mmol aryl halide, 1.4 mmol dithiophen-3-ylzinc, 4 ml NMP, catalyst (0.01 mol%) added in solution (dioxane), reaction performed at 100°C. Conversions determined by GC/MS, based on aryl halide.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article