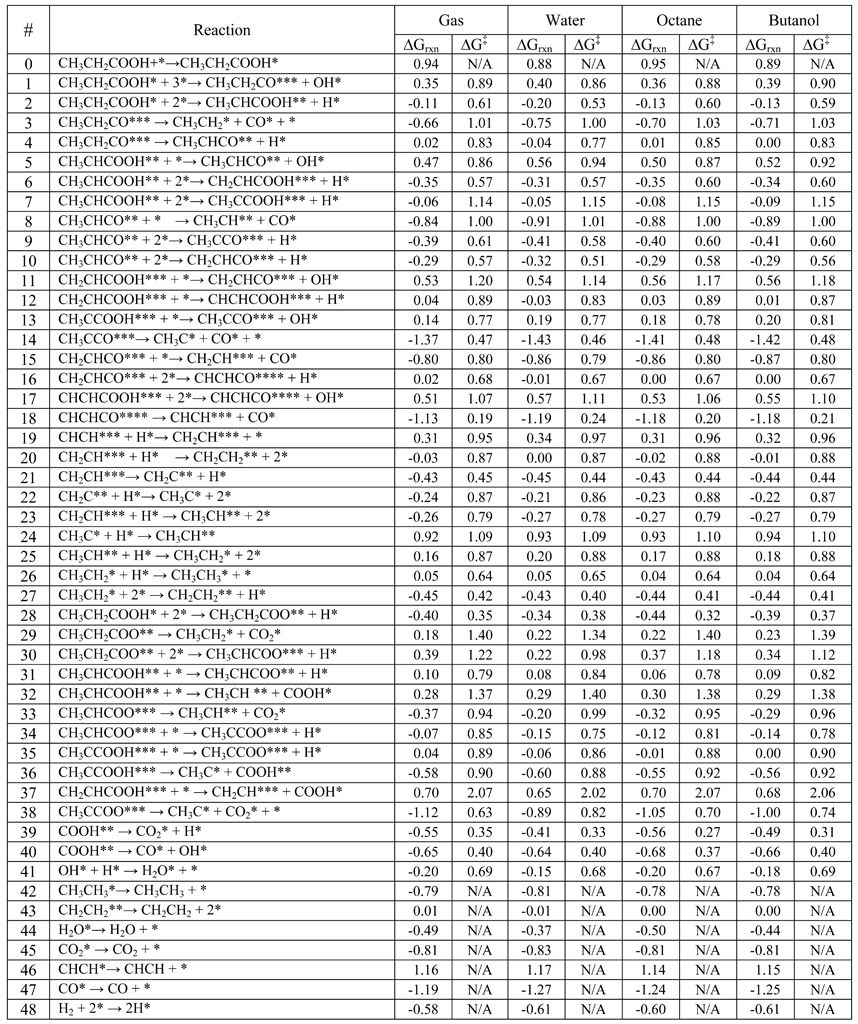
Table 1 Reaction free energies in eV for all elementary reaction steps in the hydro-deoxygenation of propanoic acid over Pd(111) model surfaces at a temperature of 473 K
Additions and corrections
Solvent effects on the hydrodeoxygenation of propanoic acid over Pd(111) model surfaces
Sina Behtash, Jianmin Lu, Muhammad Faheem and Andreas Heyden
Green Chem., 2014, 16, 605–616 (DOI: 10.1039/C3GC41368C). Amendment published 1st April 2014.
Some information was incorrect in Table 1 (p.608) in the final published article.
The values of the free energies of activation (∆G‡) are incorrect in Table 1. Entries for reaction 47 and 48 are also missing. Previously reported values for every elementary reaction are erroneously shifted by a constant value.
The authors note that this shift does not affect the results and discussion as the discussion focuses exclusively on the differences of the free energies in different solvents that are independent of the constant shift.
Furthermore, the rate constants used in Table 2 and the microkinetic model were computed with the correct values of ∆G‡.
Please find below a corrected version of Table 1.

Table 1 Reaction free energies in eV for all elementary reaction steps in the hydro-deoxygenation of propanoic acid over Pd(111) model surfaces at a temperature of 473 K
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article