DOI: 10.1039/b102650j

Paper
| Geochemical Transactions,
2001, 3 DOI: 10.1039/b102650j |

Paper |
The nature and fate of natural resins in the geospherePart XI.† Ruthenium tetroxide oxidation of a mature Class Ib amber polymer |
Ken B. Anderson
| Chemistry Division, Argonne National Laboratory, Argonne, IL, 60439, USA E-mail: kbanderson@anl.gov |
Received 22nd March 2001 , Accepted 4th June 2001
Published on the web 28th June 2001
The results of ruthenium tetroxide (RuO4) oxidation of a mature Class Ib amber polymer are reported and discussed. These data indicate that the residual double bond present in mature Class I ambers is not located in the A/B ring structure of these materials and that C17 of the original labdanoid precursors is retained in mature Class I ambers as a methyl group. These data also suggest that the reaction which results in formation of the residual unsaturated structure in mature ambers also results in a second covalent connection between the A/B ring system and the polymer backbone, probably through C8 of the original labdanoid structure.
Fossil resins (ambers), are common sedimentary constituents in many
areas, and occur in great abundance in some deposits, (e.g.,
around the Baltic region, where amber has been commercially produced for
centuries and in the Dominican Republic where commercial deposits are also
mined). The majority of significant deposits are Tertiary but older
deposits, stretching back to at least the Triassic, are known.

 Fossil resins, (ambers) are
common sedimentary constituents in many areas, and occur in great
abundance in some deposits, (e.g., around the Baltic region, where
amber has been commercially produced for centuries and in the Dominican
Republic where commercial deposits are also mined). The majority of
significant deposits are Tertiary, but older deposits stretching back to
at least the Triassic are known.
Fossil resins, (ambers) are
common sedimentary constituents in many areas, and occur in great
abundance in some deposits, (e.g., around the Baltic region, where
amber has been commercially produced for centuries and in the Dominican
Republic where commercial deposits are also mined). The majority of
significant deposits are Tertiary, but older deposits stretching back to
at least the Triassic are known.
 Ambers are classified
chemically on the basis of their molecular structure.2,3,4
Class I ambers are derived from higher plant resins based primarily on
polymers of labdatriene
diterpenes. When the plant exudes resin, (usually in response to injury or
stress of some form), these compounds undergo polymerization across the
terminal double bond located in the side chain to give a general
14,15-poly(labdatriene) polymer
structure5 (numbering used
is the accepted numbering of labdanoid
diterpenes). In many cases, a variety of related labdanoid
precursors are incorporated into the developing polymeric structure
resulting in a final copolymeric structure incorporating labdanoid
carboxylic acids, alcohols and hydrocarbons. Ambers based on both regular
and enantio labdanoids
are well known in the geosphere,6
and sub-classification of Class I ambers is based on this distinction (and
on the incorporation, or lack thereof, of
succininc acid).2
Ambers are classified
chemically on the basis of their molecular structure.2,3,4
Class I ambers are derived from higher plant resins based primarily on
polymers of labdatriene
diterpenes. When the plant exudes resin, (usually in response to injury or
stress of some form), these compounds undergo polymerization across the
terminal double bond located in the side chain to give a general
14,15-poly(labdatriene) polymer
structure5 (numbering used
is the accepted numbering of labdanoid
diterpenes). In many cases, a variety of related labdanoid
precursors are incorporated into the developing polymeric structure
resulting in a final copolymeric structure incorporating labdanoid
carboxylic acids, alcohols and hydrocarbons. Ambers based on both regular
and enantio labdanoids
are well known in the geosphere,6
and sub-classification of Class I ambers is based on this distinction (and
on the incorporation, or lack thereof, of
succininc acid).2
 To further elucidate the
details of this process, a mature Class Ib amber (Upper Cretaceous,
Yantardakh Hill region, Siberia) has been subjected to ruthenium tetroxide
(RuO4) oxidation. This reagent aggressively
cleaves carbon–carbon double bonds but does not attack saturated
structures. In order to simplify interpretation of the resulting data, the
sample was first extracted to isolate a soluble polymer phase11
which was as free as possible of extraneous occluded materials. The
results of these investigations are reported herein.
To further elucidate the
details of this process, a mature Class Ib amber (Upper Cretaceous,
Yantardakh Hill region, Siberia) has been subjected to ruthenium tetroxide
(RuO4) oxidation. This reagent aggressively
cleaves carbon–carbon double bonds but does not attack saturated
structures. In order to simplify interpretation of the resulting data, the
sample was first extracted to isolate a soluble polymer phase11
which was as free as possible of extraneous occluded materials. The
results of these investigations are reported herein.
 1 (v/v) CH2Cl2
1 (v/v) CH2Cl2
 n-pentane to remove occluded
materials. After 24 h, extraction was stopped and the CH2Cl2–n-pentane
solution removed and replaced with CH2Cl2
n-pentane to remove occluded
materials. After 24 h, extraction was stopped and the CH2Cl2–n-pentane
solution removed and replaced with CH2Cl2
 CH3OH
(1
CH3OH
(1  1 v/v) and the extraction
recommenced. After a further 24 h, the CH2Cl2–CH3OH
soluble phase was rotary evaporated to near dryness, then taken up to the
extent possible in 10 mL CH3OH and fully
precipitated by slow addition of cold H2O. The
resulting precipitate was recovered by filtration and washed with cold H2O.
Residual insoluble materials were also collected, and washed with CH3OH–H2O.
Both products were then dried overnight in vacuo at 60 °C to
give 149.6 mg soluble polymer (60% yield relative to original amber) and
15.2 mg insoluble residue (6% yield relative to original amber).
1 v/v) and the extraction
recommenced. After a further 24 h, the CH2Cl2–CH3OH
soluble phase was rotary evaporated to near dryness, then taken up to the
extent possible in 10 mL CH3OH and fully
precipitated by slow addition of cold H2O. The
resulting precipitate was recovered by filtration and washed with cold H2O.
Residual insoluble materials were also collected, and washed with CH3OH–H2O.
Both products were then dried overnight in vacuo at 60 °C to
give 149.6 mg soluble polymer (60% yield relative to original amber) and
15.2 mg insoluble residue (6% yield relative to original amber). 3 v/v 40 mL) filtered over MgSO4,
evaporated to dryness, washed again with H2O,
then finally recovered by filtration and dried at 60 °C overnight.
Yield ca. 70% (by mass).
3 v/v 40 mL) filtered over MgSO4,
evaporated to dryness, washed again with H2O,
then finally recovered by filtration and dried at 60 °C overnight.
Yield ca. 70% (by mass). 1 vol.
1 vol.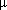 m phase
thickness), Tinit = 40 °C (4 min),
ramp at 4° min
m phase
thickness), Tinit = 40 °C (4 min),
ramp at 4° min 1,
Tfinal = 310 °C (16 min). For
analysis of the isolated polymer, TMAH was added as described elsewhere.12
No TMAH was used in Py-GC-MS analyses of the RuO4
oxidation product of the soluble polymer.
1,
Tfinal = 310 °C (16 min). For
analysis of the isolated polymer, TMAH was added as described elsewhere.12
No TMAH was used in Py-GC-MS analyses of the RuO4
oxidation product of the soluble polymer. GC-MS analyses were conducted
using an HP 5972 MSD coupled with an HP 5890 (II) equipped with an automated
direct on-column injector. GC-MS analyses reported herein (Data set 1) were
carried out using a 60 m DB-5 MS column (0.25 mm id, 0.25
GC-MS analyses were conducted
using an HP 5972 MSD coupled with an HP 5890 (II) equipped with an automated
direct on-column injector. GC-MS analyses reported herein (Data set 1) were
carried out using a 60 m DB-5 MS column (0.25 mm id, 0.25
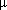 m phase
thickness) programmed as follows: Tinit
= 140 °C (4 min), ramp at 4° min
m phase
thickness) programmed as follows: Tinit
= 140 °C (4 min), ramp at 4° min 1,
Tfinal = 300 °C (10 min).
1,
Tfinal = 300 °C (10 min).13C NMR analysis of the soluble polymer
prior to oxidation indicates terminal
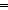 CH2
CH2 total
other C
total
other C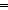 C (excluding aromatic
C) of approximately 1
C (excluding aromatic
C) of approximately 1  7.5,
indicating that maturation related transformation of the initial
exomethylene double bond is advanced to essentially complete in this
sample. (Click
here to access these data—Chime plug-in required). 1H
NMR spectra for this sample are also consistent with this conclusion. (Click
here to access these data—Chime plug-in required).
7.5,
indicating that maturation related transformation of the initial
exomethylene double bond is advanced to essentially complete in this
sample. (Click
here to access these data—Chime plug-in required). 1H
NMR spectra for this sample are also consistent with this conclusion. (Click
here to access these data—Chime plug-in required).
Results of Py-GC-MS analyses of the isolated soluble polymer of the amber used in this study are given in IDS-2.
 All of the products observed
in these data belong to one of the series of
regular bicyclic compounds
derived from the A/B ring system of various regular labdanoid
monomers. These compounds are characteristic of the pyrolysates of Class
Ia and Ib ambers.2,6
Structures in which C11 is retained predominate over those in which this
carbon is absent, as is typical for mature Class I ambers.2
These data are, not surprisingly, closely comparable with data from
analyses of the whole amber from this site,3
the only significant difference being the presence in significant
abundance of bicyclic methyl ethers, which are undoubtedly an artefact of
the isolation procedure used. These data also show that this product is
free of occluded materials, especially abietane-type diterpenes, which are
observed in the pyrolysate of the whole amber3
and which may have complicated interpretation of subsequent data from RuO4
oxidation studies.
All of the products observed
in these data belong to one of the series of
regular bicyclic compounds
derived from the A/B ring system of various regular labdanoid
monomers. These compounds are characteristic of the pyrolysates of Class
Ia and Ib ambers.2,6
Structures in which C11 is retained predominate over those in which this
carbon is absent, as is typical for mature Class I ambers.2
These data are, not surprisingly, closely comparable with data from
analyses of the whole amber from this site,3
the only significant difference being the presence in significant
abundance of bicyclic methyl ethers, which are undoubtedly an artefact of
the isolation procedure used. These data also show that this product is
free of occluded materials, especially abietane-type diterpenes, which are
observed in the pyrolysate of the whole amber3
and which may have complicated interpretation of subsequent data from RuO4
oxidation studies.
Data from Py-GC-MS analysis of the RuO4 oxidation product of the amber polymer described above, are given in IDS-3.
 Although not all of the
observed products can be unambiguously assigned at this time, data from
the hydrolysis–methylation of sclareolide (Data set 1) and comparison
of the data given in Data set 3 with literature data14
do allow the majority of products, including most of the major products,
to be assigned with a high degree of confidence. All of these compounds
(Data set 3, 1–3 and 6–8) belong to two
homologous series of compounds
which are clearly derived from, and which preserve the A/B ring of the
original labdanoids.
Although not all of the
observed products can be unambiguously assigned at this time, data from
the hydrolysis–methylation of sclareolide (Data set 1) and comparison
of the data given in Data set 3 with literature data14
do allow the majority of products, including most of the major products,
to be assigned with a high degree of confidence. All of these compounds
(Data set 3, 1–3 and 6–8) belong to two
homologous series of compounds
which are clearly derived from, and which preserve the A/B ring of the
original labdanoids.
 The structural
characteristics of these compounds are very informative in a number of
ways. Firstly, as just noted, these compounds all preserve intact the
original labdanoid A/B ring structure. Given the high yield recovered from
the oxidation procedure, this indicates that the residual double bond
present in this amber is not located in this ring system, since had it
been so, these rings would have been cleaved by the oxidation procedure.
It can also be concluded that the final double bond is not directly
attached to this ring system since if it were then one would anticipate
keto products at the point of attachment, and no such products have been
identified.
The structural
characteristics of these compounds are very informative in a number of
ways. Firstly, as just noted, these compounds all preserve intact the
original labdanoid A/B ring structure. Given the high yield recovered from
the oxidation procedure, this indicates that the residual double bond
present in this amber is not located in this ring system, since had it
been so, these rings would have been cleaved by the oxidation procedure.
It can also be concluded that the final double bond is not directly
attached to this ring system since if it were then one would anticipate
keto products at the point of attachment, and no such products have been
identified.
 It is also informative that
all of these products are
themselves unsaturated. Clearly this is a result of the pyrolytic analyses
used in this investigation since olefinic structures are very susceptible
to cleavage by RuO4 and NMR analyses of this
product prior to pyrolysis confirm the absence of C
It is also informative that
all of these products are
themselves unsaturated. Clearly this is a result of the pyrolytic analyses
used in this investigation since olefinic structures are very susceptible
to cleavage by RuO4 and NMR analyses of this
product prior to pyrolysis confirm the absence of C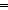 C
unsaturation. All of these products are unsaturated around
C8.
This suggests that prior to pyrolysis (and after oxidation) this carbon is
likely to be functionalized and that on pyrolysis this functionality is
being lost, probably through dehydration or decarboxylation or a similar
mechanism.
C
unsaturation. All of these products are unsaturated around
C8.
This suggests that prior to pyrolysis (and after oxidation) this carbon is
likely to be functionalized and that on pyrolysis this functionality is
being lost, probably through dehydration or decarboxylation or a similar
mechanism.
 In all of these products
C17 of
the original labdanoid
is
retained. In the original labdanoid structure this carbon is present
in an exomethylene structure. Progressive
In all of these products
C17 of
the original labdanoid
is
retained. In the original labdanoid structure this carbon is present
in an exomethylene structure. Progressive
 loss
loss of exomethylene character is one of the defining characteristics of
maturation of these polymers,2,7,8,9
and it might be (and in fact has been8,9,15
) postulated that this reflects cross-linking or condensation reactions
directly involving this structure. However, the preservation of this
carbon in all cases as a methyl group argues against this possibility
since such reactions would retain this carbon as a methylene structure and
it is difficult to postulate any mechanism by which such a structure can
be universally converted to a methyl group upon pyrolysis. It is also
noteworthy that all of the characteristic
bicyclic structures observed in the pyrolysates of Class I ambers
also retain
this
carbon as a methyl group, which supports the view that this carbon
is retained as a methyl group in mature Class I ambers.
of exomethylene character is one of the defining characteristics of
maturation of these polymers,2,7,8,9
and it might be (and in fact has been8,9,15
) postulated that this reflects cross-linking or condensation reactions
directly involving this structure. However, the preservation of this
carbon in all cases as a methyl group argues against this possibility
since such reactions would retain this carbon as a methylene structure and
it is difficult to postulate any mechanism by which such a structure can
be universally converted to a methyl group upon pyrolysis. It is also
noteworthy that all of the characteristic
bicyclic structures observed in the pyrolysates of Class I ambers
also retain
this
carbon as a methyl group, which supports the view that this carbon
is retained as a methyl group in mature Class I ambers.
 If it is correct that
C17 is
retained in mature Class I ambers as a methyl group, then this implies
that isomerization of the initial exomethylene structure is an early step
in the maturation of these materials. However, as noted above, the data
given in Data set 3 preclude the presence of a double bond in, or on the
labdanoid A/B ring system in mature samples. How then, can these
observations be reconciled? One additional observation is helpful in this
regard—the product of RuO4 oxidation of
this polymer was not amenable to analysis by GC-MS. Although it was not
possible to determine absolute molecular weights for the RuO4
oxidation products described herein, this observation suggests that this
product is still a high molecular weight material.
If it is correct that
C17 is
retained in mature Class I ambers as a methyl group, then this implies
that isomerization of the initial exomethylene structure is an early step
in the maturation of these materials. However, as noted above, the data
given in Data set 3 preclude the presence of a double bond in, or on the
labdanoid A/B ring system in mature samples. How then, can these
observations be reconciled? One additional observation is helpful in this
regard—the product of RuO4 oxidation of
this polymer was not amenable to analysis by GC-MS. Although it was not
possible to determine absolute molecular weights for the RuO4
oxidation products described herein, this observation suggests that this
product is still a high molecular weight material.
 If maturation of polymers
with an initial 14,15-poly(labdatriene)
structure results in a structure with one double bond, which is not
located in nor directly attached to the A/B ring systems of the labdanoid
monomers, then it follows ex necessitate rei, that the double bond
must be located somewhere in the side chain structure. However, one would
expect oxidation of such a structure to give volatile, low molecular
weight products due to cleave of the linkage between the polymer backbone
and the A/B ring system, and this is observed on oxidation of immature
samples (Anderson, unpublished results). The absence of volatile products
from the RuO4 oxidation of this mature sample
therefore suggests that covalent connection still exists between the
polymer backbone and the A/B ring system after oxidation.
If maturation of polymers
with an initial 14,15-poly(labdatriene)
structure results in a structure with one double bond, which is not
located in nor directly attached to the A/B ring systems of the labdanoid
monomers, then it follows ex necessitate rei, that the double bond
must be located somewhere in the side chain structure. However, one would
expect oxidation of such a structure to give volatile, low molecular
weight products due to cleave of the linkage between the polymer backbone
and the A/B ring system, and this is observed on oxidation of immature
samples (Anderson, unpublished results). The absence of volatile products
from the RuO4 oxidation of this mature sample
therefore suggests that covalent connection still exists between the
polymer backbone and the A/B ring system after oxidation.
 Hence, we are left with the
following:
Hence, we are left with the
following:
 (i) Maturation of Class I
ambers results in a structure with
(i) Maturation of Class I
ambers results in a structure with
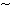 1 double bond per original
monomer.8,9
1 double bond per original
monomer.8,9
 (ii) This residual double bond
is not structurally equivalent to either of the initial double bonds,10
nor is it located in, or directly attached to the labdanoid A/B ring
system of the original monomer.
(ii) This residual double bond
is not structurally equivalent to either of the initial double bonds,10
nor is it located in, or directly attached to the labdanoid A/B ring
system of the original monomer.
 (iii)
C17 of
the original labdanoid structure is retained in the mature structure as a
methyl group.
(iii)
C17 of
the original labdanoid structure is retained in the mature structure as a
methyl group.
 (iv) In addition to the
connection through the original side chain, maturation results in a new
covalent connection between the polymer backbone and the labdanoid A/B
ring system.
(iv) In addition to the
connection through the original side chain, maturation results in a new
covalent connection between the polymer backbone and the labdanoid A/B
ring system.
The support of the US Department of Energy, Office of Science. Basic
Energy Sciences Division of Chemical Sciences, Geosciences, and
Biosciences, under contract number W-31-109-ENG-38 is gratefully
acknowledged. Dr. John V. Muntean (Chemistry Division, Argonne National
Laboratory) is thanked for his assistance in obtaining the NMR data
described in this report. Chemical structures are rendered in this report
using the MarvinView( ) Java
Applet developed by Peter Csizmadia and coworkers at ChemAxon Ltd.
Systematic names for structures reported herein are generated using
AutoNom2000 (MDL Inc.) using CAS naming conventions. The comments of two
anonymous reviewers are also gratefully acknowledged.
) Java
Applet developed by Peter Csizmadia and coworkers at ChemAxon Ltd.
Systematic names for structures reported herein are generated using
AutoNom2000 (MDL Inc.) using CAS naming conventions. The comments of two
anonymous reviewers are also gratefully acknowledged.
† For Part X of this series see ref. 1.