Additions and corrections
Synthesis and charge-transfer complexes of a new donor molecule, TP-EDOT
Hideki Yamochi, Jun Hagiwara, Masaya Soeda and Gunzi Saito
J. Mater. Chem., 2006, 16, 550–557 (DOI: 10.1039/b511512d). Amendment published 14th August 2006.
The authors would like to draw the readers' attention to the following points:
(1) In the original version of the article, the 2nd sentence from the last in Fig. 6 caption is incorrect. The following sentence indicates the corrected intermolecular overlap integrals:
The numerical values are; s1 = -23.02, s2 = -16.14, s3 = -3.42, s4 = s4' = 0.92, s5 = s6 = ca. 0.1 x 10-3.
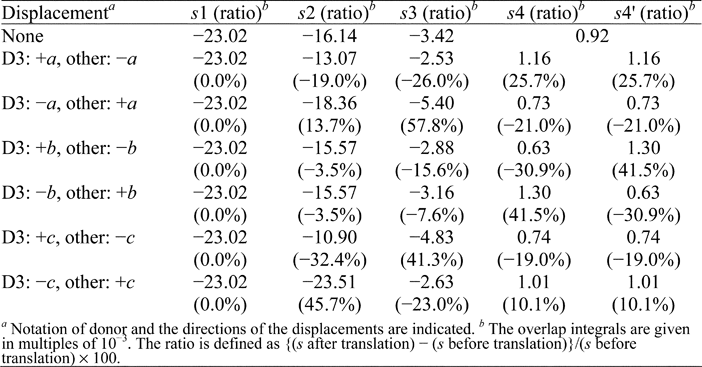
(2) The molecular displacements and numerical values in Table 2 are incorrect. The corrected version of this table is shown below:
Table 2 Variation of the intermolecular overlap integrals by 0.1 Å displacements of the donor molecules in (TP-EDOT)3Sb2F11(benzene)

(3) In the subsection 'Origin of the diamagnetic semiconducting behaviour' starting on p. 556 in the original publication, the second and sixth paragraphs are incorrect since the descriptions were based on the wrong data. The corrected paragraphs are given below, in which the bold type indicates the corrected parts.
(3a) The 2nd paragraph should be replaced by the following sentences:
The temperature factor of 0.05 Å2, which is usually applied to the initial value for crystal structure analyses, corresponds to the mean value of the atomic displacement of 0.22 Å. To deal with the slight displacement of the molecules, we restricted the molecular motions within this range. The intra-trimer overlap integrals (s1 and s2) and the most significant inter-trimer ones of s3, s4, and s4' were calculated supposing D3 and other donor molecules were displaced by 0.1 Å in opposite directions to each other. Table 2 lists the calculated overlap integrals after the displacement of the donor molecules along a-, b-, and c-axes, which correspond to the molecular longitudinal, lateral, and stacking directions, respectively, along with the values before the translation. The 0.1 Å translation of D3 and other donor molecules along the c-axis in reverse directions to each other resulted in the effective modulation of 41.3% for the inter-trimer overlap integral of s3. Also, the modulations of s4 and s4' were noteworthy when the displacements along the b-axis were assumed. These overlap integrals were enhanced by 41.5% in magnitude. The biggest difference between before and after the displacement was observed on s2 for the molecular translation along c-axis (D3: -c, other: +c) as 7.37 x 10-3. This modulation, however, may have little effect on the electronic structure since the original magnitude of s2 is around 4.7 times bigger than that of the inter-trimer overlap integrals. In (TP-EDOT)3Sb2F11(benzene), a unit charge is encapsulated in a donor trimer, which interacts weakly with the neighbouring ones. The magnitudes of inter-trimer interactions are very sensitive to the molecular displacements as demonstrated above. In fact, assuming the slightly bigger molecular displacement of 0.15 Å along the unit cell diagonal direction (D3: a - c, other: c - a) provided the ratio of 1.14 for |s3|/|s4| when sulfur 3d orbitals were taken into account. The slight translation of the donor molecule will provide the diamagnetic dimer of the trimers.
(3b) The 6th paragraph should be replaced by the following sentences:
As described in the introductory part of this report, a BEDO-TTF has two neighbouring donor molecules in each of 0, 30, and 60º directions in the self-assembled crystal structures. As summarized in Table S2 in ESI,† the most effective modulation of 48.8% was calculated for the intermolecular overlap integrals along the 0º direction, which simultaneously showed the biggest difference between the intermolecular overlap integrals before and after the displacement (1.95 x 10-3). The variations of intermolecular overlap integrals according to the molecular displacements have the same order of magnitude in both complexes. The distinct feature of the (BEDO-TTF)5(HCTMM)(benzonitrile)2 is that this complex always preserves the 2D electronic structure regardless of the directions of the molecular displacements. This insensitivity should provide the strong 2D nature of the electronic structure of this complex.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article