Additions and corrections
Surface phase diagram of hematite pseudocubes in hydrous environments
Haibo Guo and Amanda S. Barnard
J. Mater. Chem., 2012, 22, 161–167 (DOI: 10.1039/c1jm13362d). Amendment published 2nd July 2012.
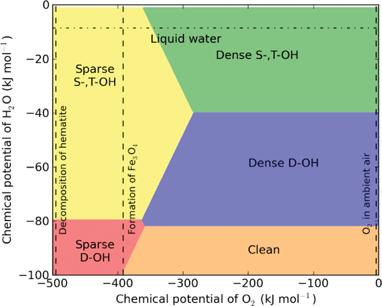
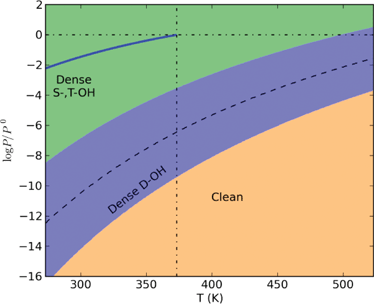
Due to mistakes in calculating the temperature-dependent chemical potential of water vapour, the phase boundaries in the phase diagrams shown in Figs. 3, 4 and 7 were incorrect. The correct figures are as follows:
Fig. 3:

Fig. 4:
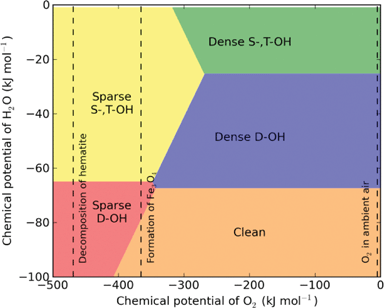
Fig. 7:
On page 166, left column: the transition temperatures in the paragraph beginning with “Pressures larger than” are corrected as follows (highlighted in bold font).
‘…For example, when pH2O = 1 atm, the transition temperature for dense S-,T-OH to dense D-OH is approximately 500 K, and for pH2O = 3.2 kPa (saturated vapour pressure of water at room temperature), the transition temperature is approximately 425 K. To initiate the transition to the clean surface, higher temperatures or lower partial pressures of water vapour are required. With pH2O = 1 atm the transition temperature from the dense D-OH to the clean surface is 695 K. Under vacuum conditions the transition temperatures will be substantially reduced. It has been reported that water molecules desorb from hematite (012) surface at 353 K measured using temperature programmed desorption under ultra-high vacuum (UHV).22,31 This desorption temperature is approximately 25 K higher than our calculations for the UHV condition (10-7 Pa or 10-12 atm), suggesting the water desorption requires an activation energy.’
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article