Additions and corrections Inhibition of the PLP-dependent enzyme serine palmitoyltransferase by cycloserine: evidence for a novel decarboxylative mechanism of inactivation |
Jonathan Lowther, Beverley A. Yard, Kenneth A. Johnson, Lester G. Carter, Venugopal T. Bhat, Marine C. C. Raman, David J. Clarke, Britta Ramakers, Stephen A. McMahon, James H. Naismith and Dominic J. Campopiano
Mol. BioSyst., 2011, DOI: 10.1039/c003743e. Amendment published 3rd December 2010
Figure 1 and the legend in the original document are incorrect. The correct version is as below:
 |
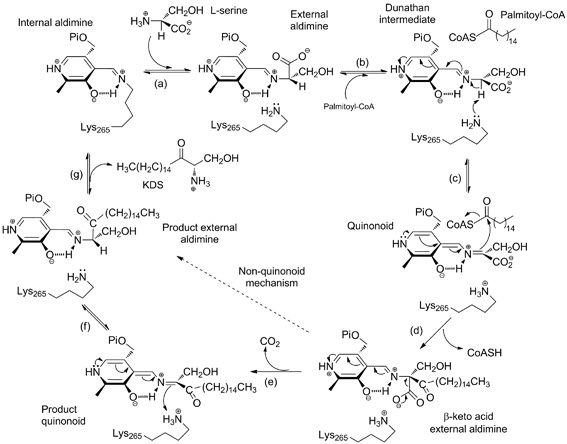
Fig. 1. Proposed catalytic mechanism of SPT. (a) The internal aldimine (holo-SPT) is displaced by L-serine to form the external adlimine; (b) binding of second substrate palmitoyl-CoA causes a conformational change to give the putative Dunathan intermediate; (c) formation of the quinonoid by deprotonation of the Ca hydrogen; (d) nucleophilic attack on the thioester by the quinonoid and release of CoASH to give the b-keto acid intermediate; (e) decarboxylation to form the KDS product quinonoid; (f) reprotonation to form the KDS product external aldimine; (g) KDS product release and reformation of the holo-SPT internal aldimine form. The dotted line shows that steps (e) and (f) may be by-passed by an alternative mechanism that uses the product ketone as an electron sink, rather than the PLP ring.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.