Additions and corrections
Cyclochiral conformers of resorcin[4]arenes stabilized by hydrogen bonds
Agnieszka Szumna
Organic & Biomolecular Chemistry, 2005, 5, 1358–1368 (DOI: 10.1039/b701451a)
Amendment published 27th April 2007
The authors regret the following error:
This reference was added by the author after publication of the article: M. Luostarinen, M. Nissinen, M. Nieger, A. Shivanyuk and K. Rissanen, Tetrahedron, 2007, 63, 1254–1263.
Amendment published 29th May 2007
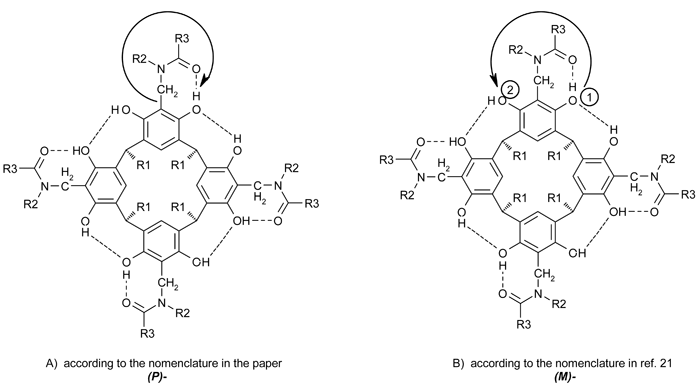
The author would like to correct and clarify issues concerning the nomenclature of stereochemistry of cyclochiral resorcinarenes, which emerged during the course of discussion with Prof. H. Heaney.
Correction: The views from above and within the cavity produce the same result, not the opposite result, as stated in the paper.
Clarification: In the paper, the stereochemistry was described consistently by the “direction of the hydrogen-bonded ring closures”. The nomenclature applied previously to covalently cyclochiral compounds used the CIP rules (ref. 21). Even though the cyclochirality of resorcinarenes presented in the paper is of non-covalent origin, the author suggests that the CIP rules can still be applied by assuming that the doubly hydrogen bonded oxygen takes the priority over the singly hydrogen bonded oxygen. In order to avoid confusion in publications in this area, the author suggests that the nomenclature described in ref. 21 is more general and thus should be applied. The approach used in the paper and that now suggested produce opposite results, and the relationship is shown in Fig. 1.

Fig. 1 The stereochemical nomenclature of conformationally cyclochiral resorcinarenes: approach B is recommended.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.